Abstract
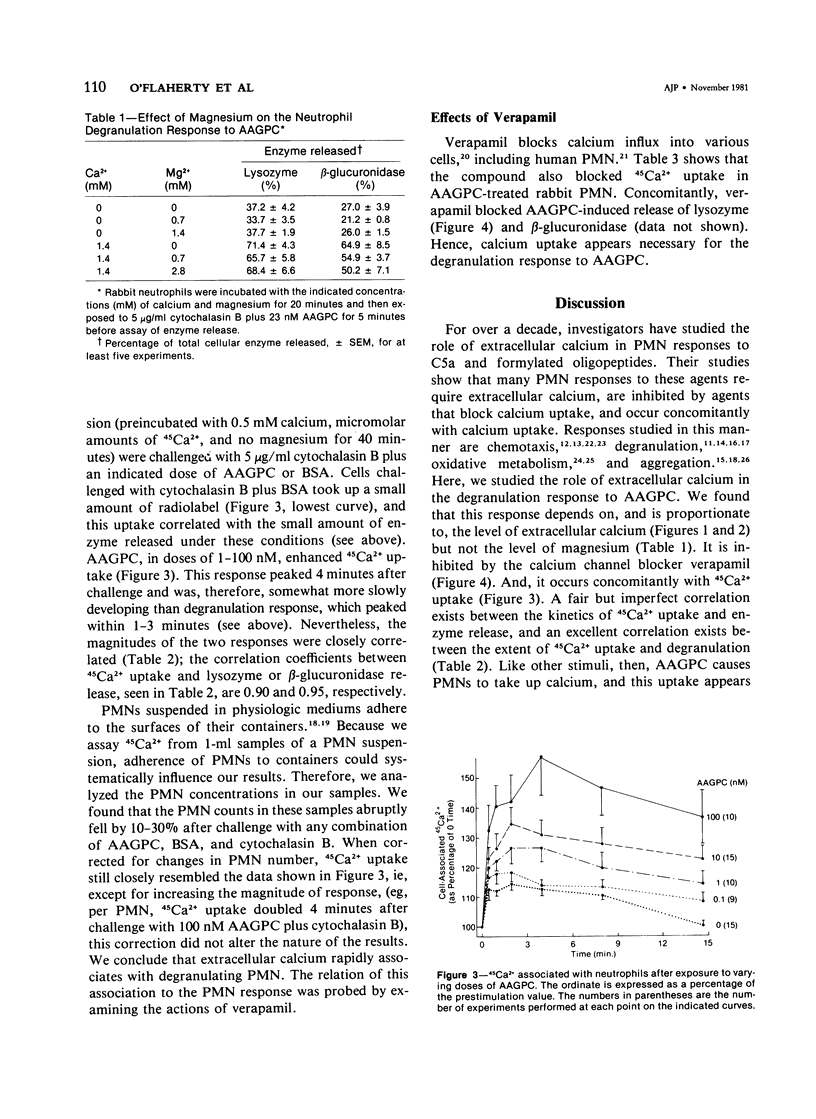
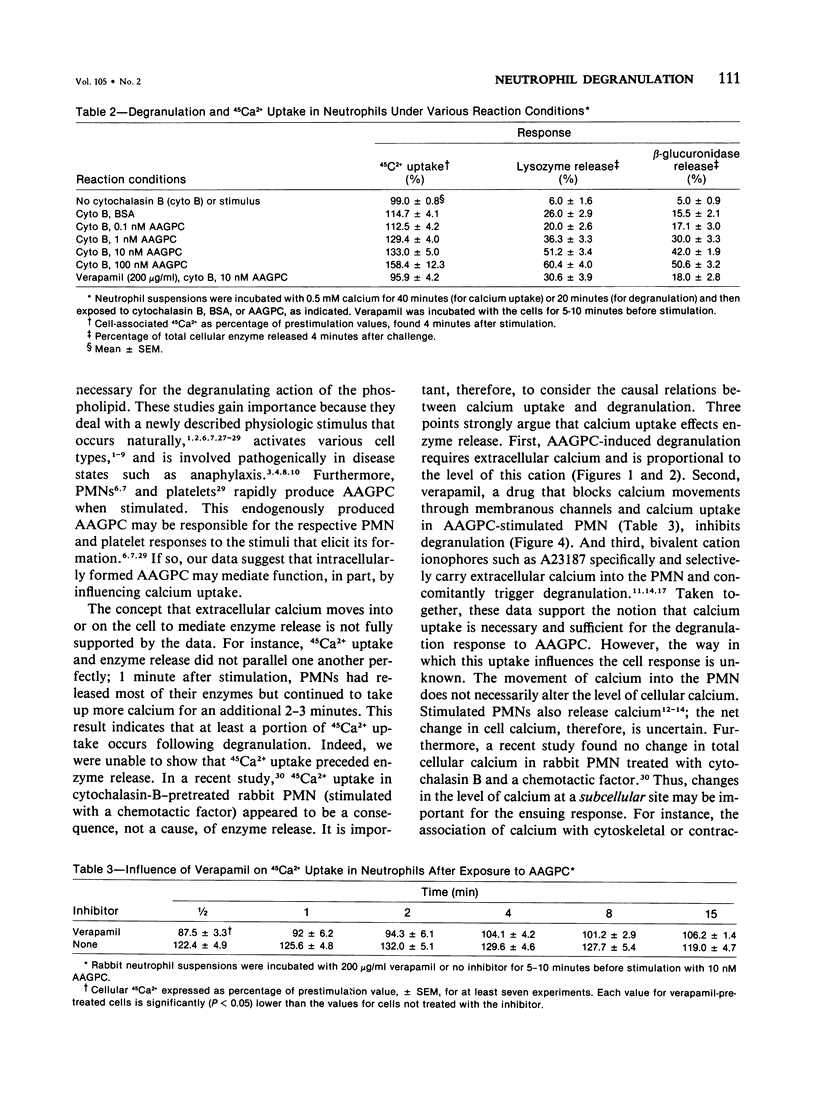
The rabbit polymorphonuclear neutrophil degranulation response to 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine depends on extracellular calcium. In the absence of this bivalent cation, neutrophil suspensions pretreated with cytochalasin B responded to the lipid by releasing minimal amounts of lysozyme and beta-glucuronidase. Incremental increases in extracellular calcium over a range of 20-200 microM led to increasing amounts of lipid-stimulated enzyme release. In contrast, extracellular magnesium neither supported nor enhanced the degranulation responses. Verapamil (25-200 microgram/ml), a calcium channel blocker, inhibited degranulation. Neutrophil suspensions exposed to the phosphocholine stimulus rapidly took up radiolabeled extracellular calcium. The kinetics of this calcium uptake were similar to the kinetics of enzyme release, and the amount of calcium taken up correlated closely with the amount of released lysozyme and beta-glucuronidase. Finally, in a dosage which blocked degranulation, verapamil inhibited calcium uptake. Thus, the rapid association of extracellular calcium with the neutrophil may mediate, at least in part, the degranulating actions of the phosphocholine stimulus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker E. L., Showell H. J. The effect of Ca2+ and Mg2+ on the chemotactic responsiveness and spontaneous motility of rabbit polymorphonuclear leukocytes. Z Immunitatsforsch Exp Klin Immunol. 1972 Jun;143(5):466–476. [PubMed] [Google Scholar]
- Benveniste J., Camussi J., Polonsky J. Platelet-activating factor. Monogr Allergy. 1977;12:138–142. [PubMed] [Google Scholar]
- Benveniste J., Tencé M., Varenne P., Bidault J., Boullet C., Polonsky J. Semi-synthèse et structure proposée du facteur activant les plaquettes (P.A.F.): PAF-acether, un alkyl ether analogue de la lysophosphatidylcholine. C R Seances Acad Sci D. 1979 Nov 26;289(14):1037–1040. [PubMed] [Google Scholar]
- Boucek M. M., Snyderman R. Calcium influx requirement for human neutrophil chemotaxis: inhibition by lanthanum chloride. Science. 1976 Sep 3;193(4256):905–907. doi: 10.1126/science.948752. [DOI] [PubMed] [Google Scholar]
- Camussi G., Bussolino F., Tetta C., Brusca R., Ragni R. The binding of platelet-activating factor (PAF) to polymorphonuclear neutrophils (PMN) as a trigger for the immune-induced PMN aggregation. 1980 Jan-Mar 22Panminerva Med. 22(1):1–5. [PubMed] [Google Scholar]
- Camussi G., Tetta C., Bussolino F., Caligaris Cappio F., Coda R., Masera C., Segoloni G. Mediators of immune-complex-induced aggregation of polymorphonuclear neutrophils. II. Platelet-activating factor as the effector substance of immune-induced aggregation. Int Arch Allergy Appl Immunol. 1981;64(1):25–41. doi: 10.1159/000232671. [DOI] [PubMed] [Google Scholar]
- Chignard M., Le Couedic J. P., Vargaftig B. B., Benveniste J. Platelet-activating factor (PAF-acether) secretion from platelets: effect of aggregating agents. Br J Haematol. 1980 Nov;46(3):455–464. doi: 10.1111/j.1365-2141.1980.tb05993.x. [DOI] [PubMed] [Google Scholar]
- Clark P. O., Hanahan D. J., Pinckard R. N. Physical and chemical properties of platelet-activating factor obtained from human neutrophils and monocytes and rabbit neutrophils and basophils. Biochim Biophys Acta. 1980 Feb 21;628(1):69–75. doi: 10.1016/0304-4165(80)90352-9. [DOI] [PubMed] [Google Scholar]
- Demopoulos C. A., Pinckard R. N., Hanahan D. J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J Biol Chem. 1979 Oct 10;254(19):9355–9358. [PubMed] [Google Scholar]
- Goetzl E. J., Derian C. K., Tauber A. I., Valone F. H. Novel effects of 1-O-hexadecyl-2-acyl-sn-glycero-3-phosphorycholine mediators on human leukocyte function: delineation of the specific roles of the acyl substituents. Biochem Biophys Res Commun. 1980 Jun 16;94(3):881–888. doi: 10.1016/0006-291x(80)91317-0. [DOI] [PubMed] [Google Scholar]
- Halonen M., Palmer J. D., Lohman I. C., McManus L. M., Pinckard R. N. Respiratory and circulatory alterations induced by acetyl glyceryl ether phosphorylcholine, a mediator of IgE anaphylaxis in the rabbit. Am Rev Respir Dis. 1980 Dec;122(6):915–924. doi: 10.1164/arrd.1980.122.6.915. [DOI] [PubMed] [Google Scholar]
- Lehmeyer J. E., Snyderman R., Johnston R. B., Jr Stimulation of neutrophil oxidative metabolism by chemotactic peptides: influence of calcium ion concentration and cytochalasin B and comparison with stimulation by phorbol myristate acetate. Blood. 1979 Jul;54(1):35–45. [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Changes in ionic movements across rabbit polymorphonuclear leukocyte membranes during lysosomal enzyme release. Possible ionic basis for lysosomal enzyme release. J Cell Biol. 1977 Dec;75(3):635–649. doi: 10.1083/jcb.75.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Transport of sodium, potassium, and calcium across rabbit polymorphonuclear leukocyte membranes. Effect of chemotactic factor. J Cell Biol. 1977 May;73(2):428–444. doi: 10.1083/jcb.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Craddock P. R., Jacob H. S. Effect of intravascular complement activation on granulocyte adhesiveness and distribution. Blood. 1978 Apr;51(4):731–739. [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Becker E. L., Ward P. A. Substances which aggregate neutrophils. Mechanism of action. Am J Pathol. 1978 Jul;92(1):155–166. [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Ward P. A. Influence of extracellular Ca2+ and Mg2+ on chemotactic factor-induced neutrophil aggregation. Inflammation. 1977 Dec;2(4):265–276. doi: 10.1007/BF00921006. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Wykle R. L., Miller C. H., Lewis J. C., Waite M., Bass D. A., McCall C. E., DeChatelet L. R. 1-O-Alkyl-sn-glyceryl-3-phosphorylcholines: a novel class of neutrophil stimulants. Am J Pathol. 1981 Apr;103(1):70–78. [PMC free article] [PubMed] [Google Scholar]
- Oseas R. S., Boxer L. A., Butterick C., Baehner R. L. Differences in polymorphonuclear leukocyte aggregating responses among several species in response to chemotactic stimulation. J Lab Clin Med. 1980 Aug;96(2):213–221. [PubMed] [Google Scholar]
- Petroski R. J., Naccache P. H., Becker E. L., Sha'afi R. I. Effect of the chemotactic factor formyl methionyl- leucyl-phenylalanine and cytochalasin B on the cellular levels of calcium in rabbit neutrophils. FEBS Lett. 1979 Apr 1;100(1):161–165. doi: 10.1016/0014-5793(79)81155-2. [DOI] [PubMed] [Google Scholar]
- Pinckard R. N., Farr R. S., Hanahan D. J. Physicochemical and functional identity of rabbit platelet-activating factor (PAF) released in vivo during IgE anaphylaxis with PAF released in vitro from IgE sensitized basophils. J Immunol. 1979 Oct;123(4):1847–1857. [PubMed] [Google Scholar]
- Racker E. Fluxes of Ca2+ and concepts. Fed Proc. 1980 May 15;39(7):2422–2426. [PubMed] [Google Scholar]
- Showell H. J., Williams D., Becker E. L., Naccache P. H., Sha'afi R. Desensitization and deactivation of the secretory responsiveness of rabbit neutrophils induced by the chemotactic peptide, formyl-methionyl-leucyl-phenylalanine. J Reticuloendothel Soc. 1979 Feb;25(2):139–150. [PubMed] [Google Scholar]
- Smith R. J., Ignarro L. J. Bioregulation of lysosomal enzyme secretion from human neutrophils: roles of guanosine 3':5'-monophosphate and calcium in stimulus-secretion coupling. Proc Natl Acad Sci U S A. 1975 Jan;72(1):108–112. doi: 10.1073/pnas.72.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]


