Abstract
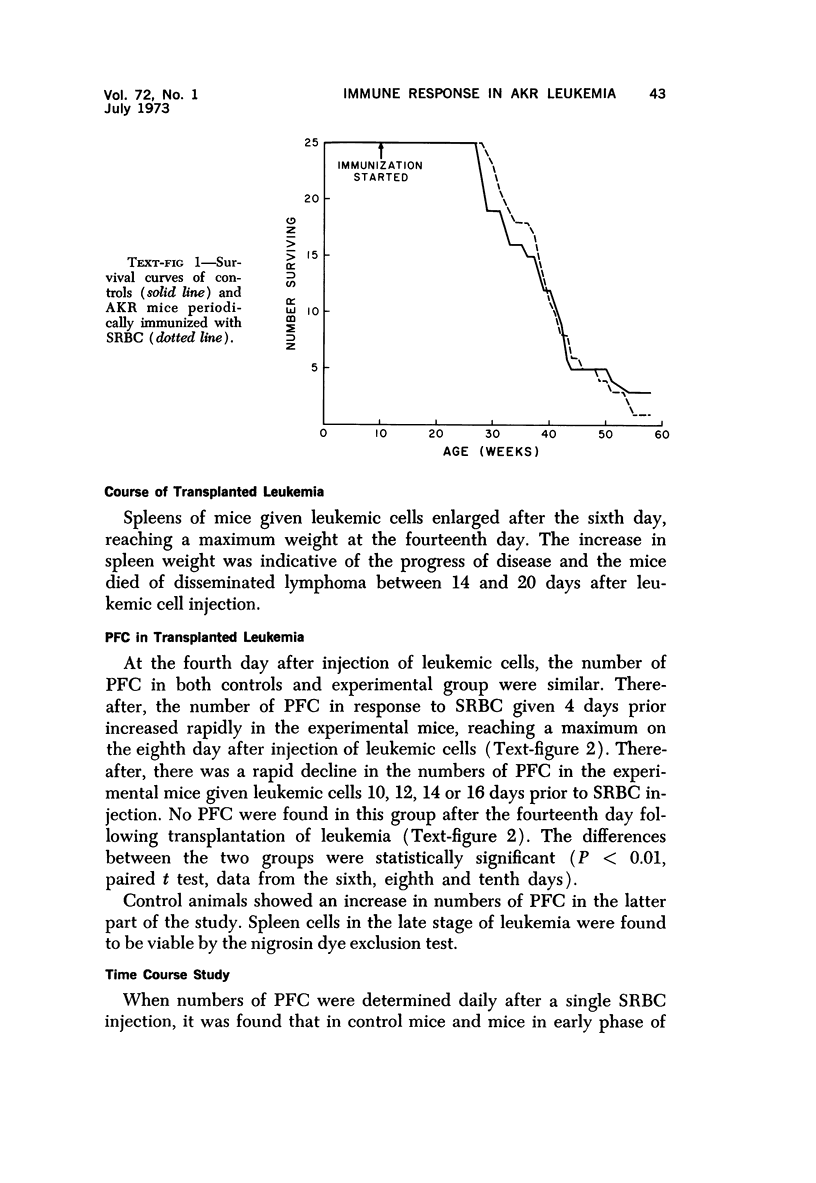
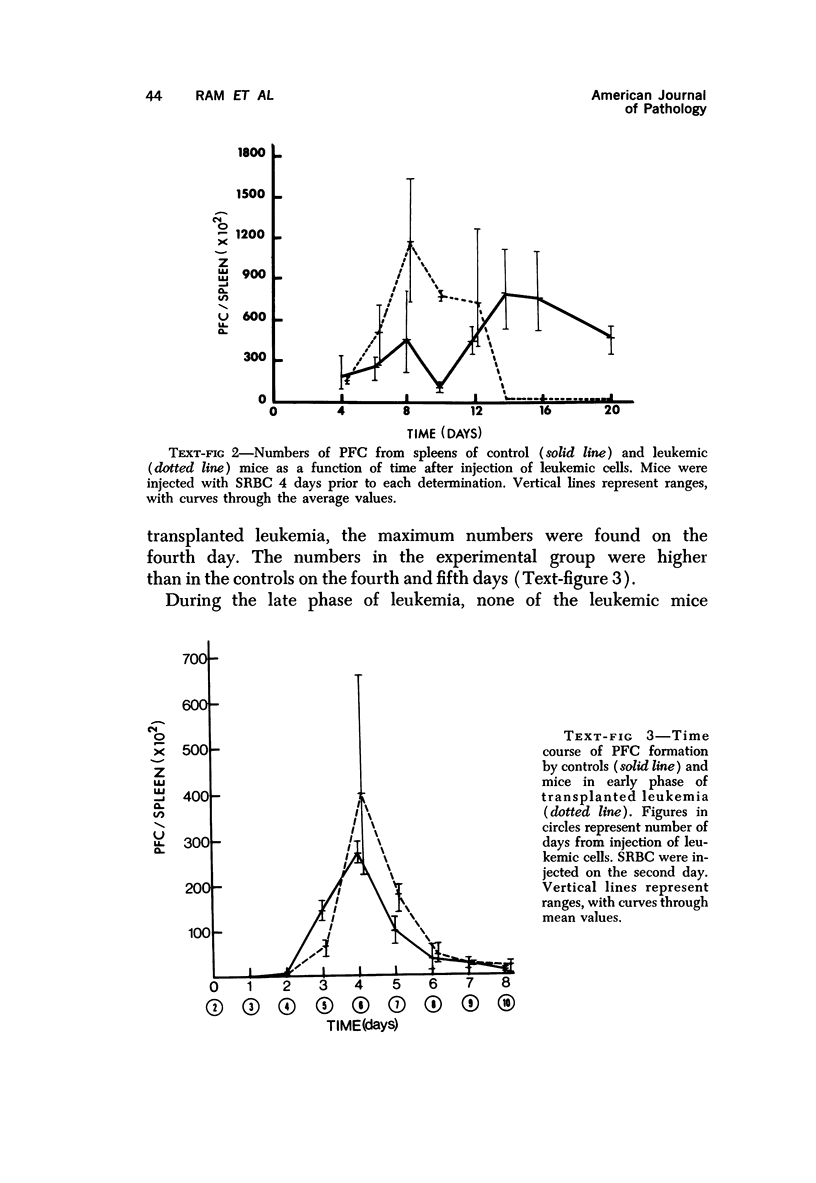
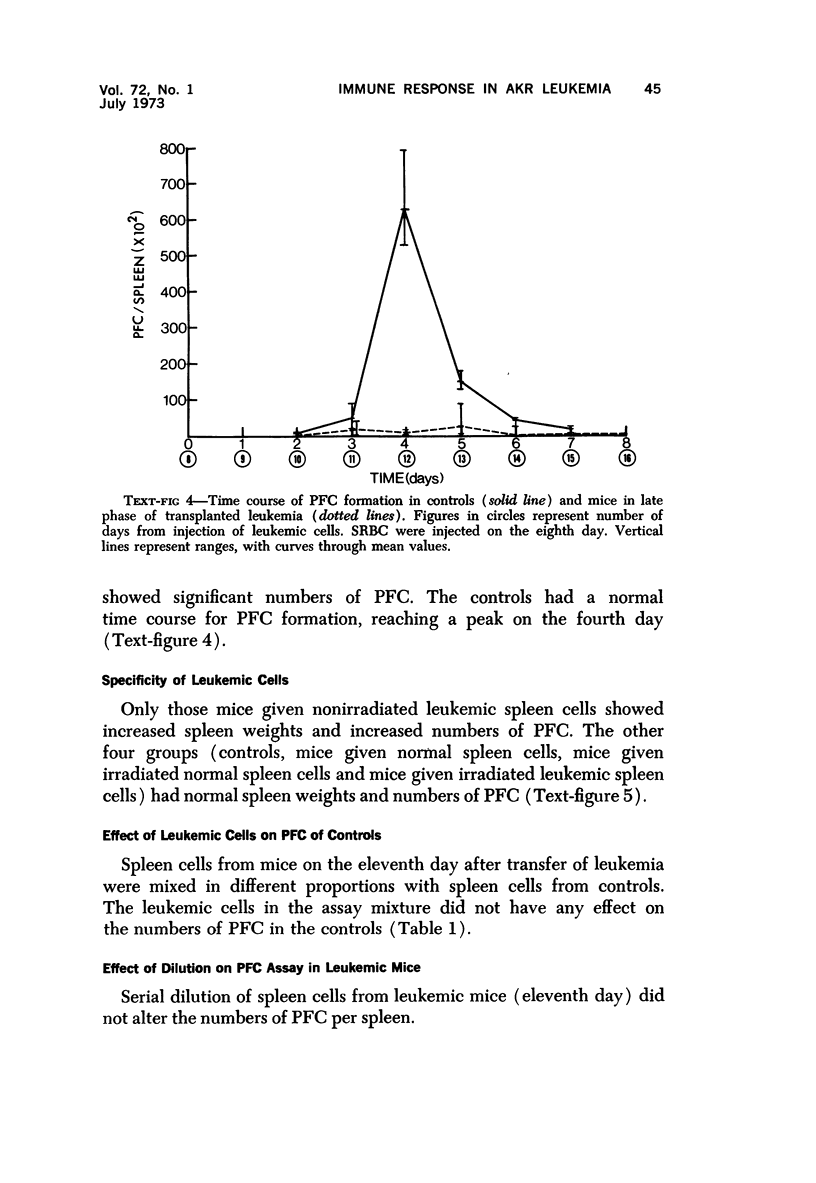
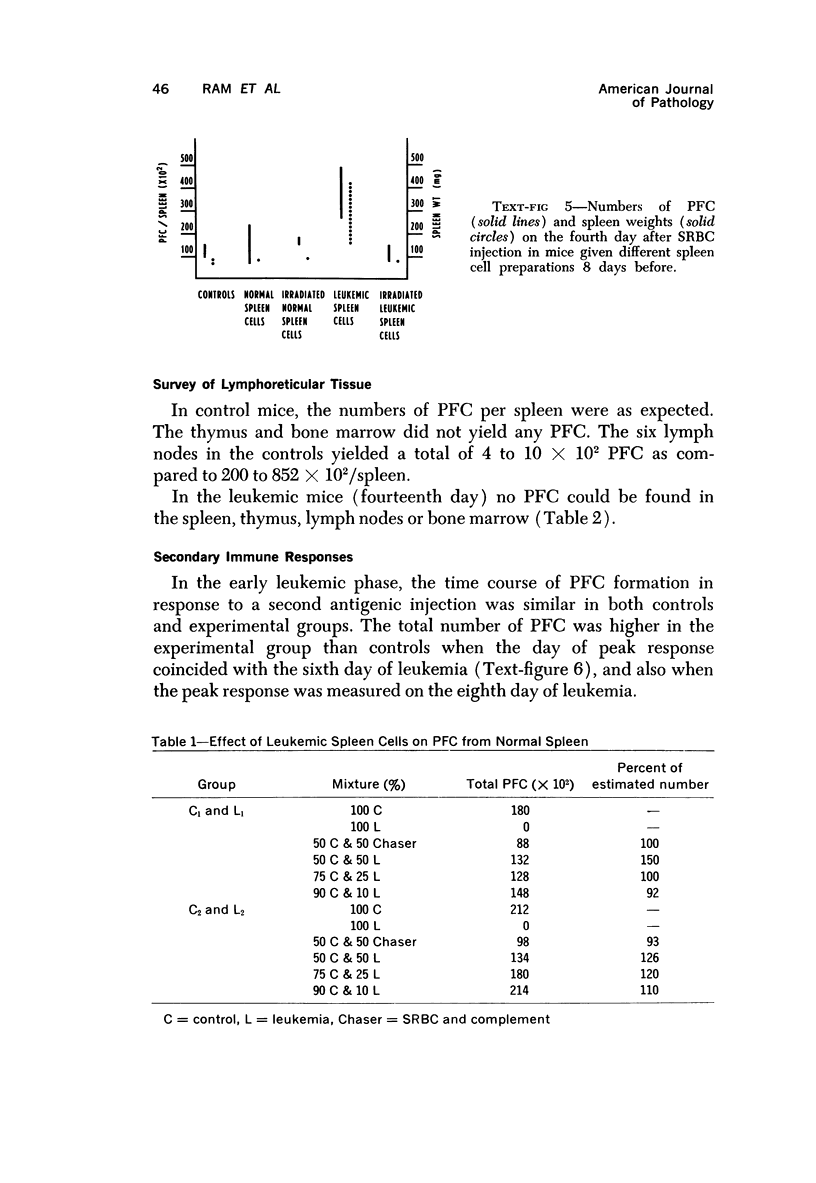
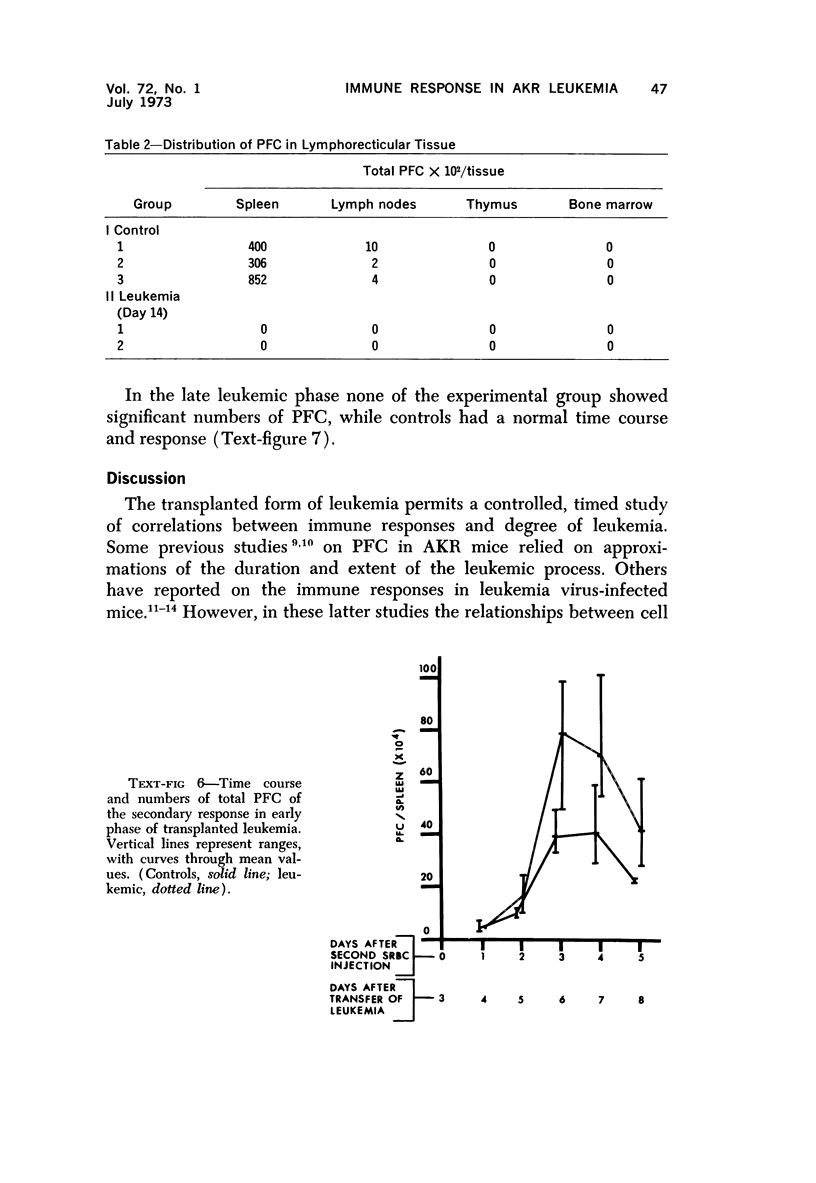
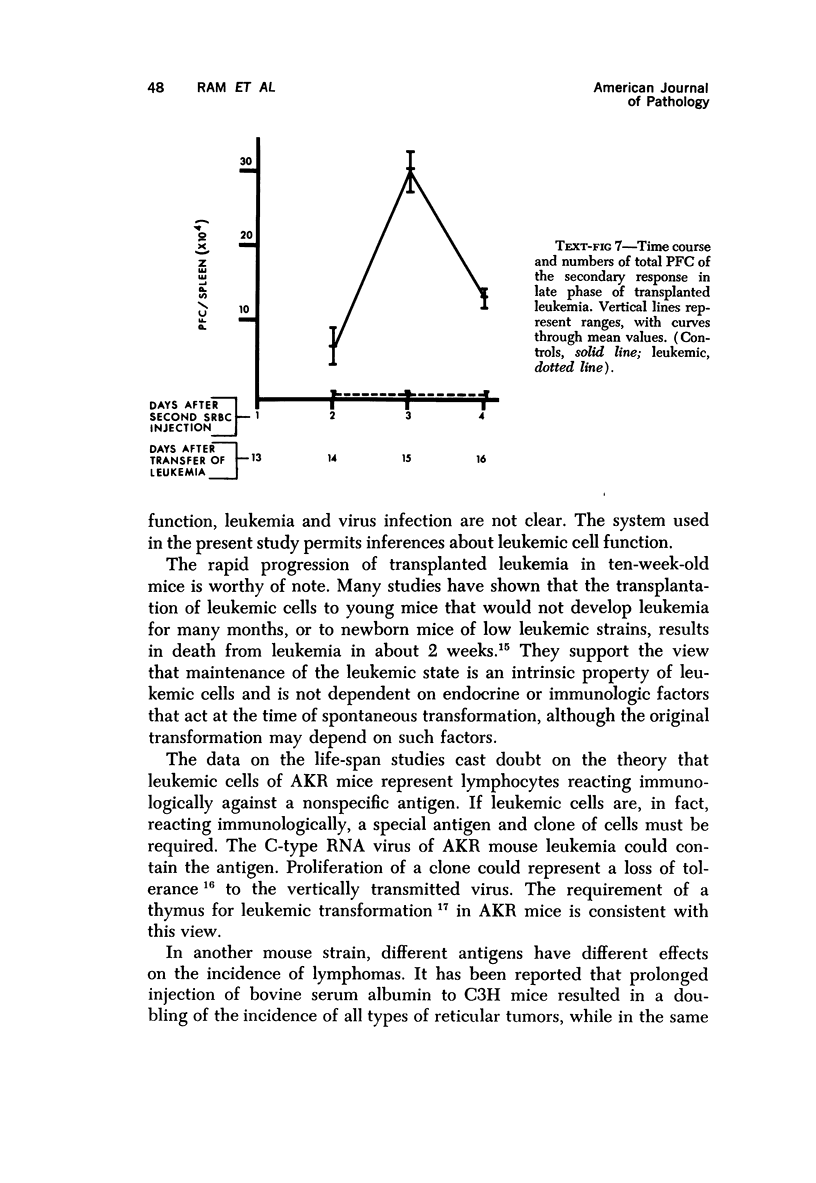
Long-term administration of sheep red cells to AKR mice did not influence the development of spontaneous leukemia. In mice with transplanted leukemia there was an increase in numbers of spleen hemolytic plaque-forming cells (PFC) during the early leukemic phase, as compared to controls, and a marked decrease and final disappearance of PFC from all lymphoid organs in the late leukemic phase. This pattern was seen in both the primary and secondary immune responses. The time course of PFC response in both the primary and the secondary response was similar to that of controls in the early leukemic phase, while there was only a negligible response without a peak in the late leukemic phase. The early increase in PFC was observed only in mice that received viable leukemic cells and not in mice injected with other cell preparations. The absence of PFC in late leukemia did not appear to be caused by an interaction between leukemic and normal cells in the assay procedure.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ceglowski W. S., Friedman H. Immunosuppression by leukemia viruses. II. Cytokinetics of appearance of hemolysin-forming cells in infected mice during the anamnestic response to sheep erythrocytes. J Immunol. 1969 Feb;102(2):338–346. [PubMed] [Google Scholar]
- Ceglowski W. S., Friedman H. Immunosuppression by leukemia viruses. IV. Effect of Friend leukemia virus on antibody-precursors as assessed by cell transfer studies. J Immunol. 1970 Dec;105(6):1406–1415. [PubMed] [Google Scholar]
- Ceglowski W. S., Friedman H. Immunosuppressive effects of Friend and Rauscher leukemia disease viruses on cellular and humoral antibody formation. J Natl Cancer Inst. 1968 May;40(5):983–995. [PubMed] [Google Scholar]
- Cole L. J., Nowell P. C. Parental-F1 hybrid bone marrow chimeras: high incidence of donor-type lymphomas. Proc Soc Exp Biol Med. 1970 Jul;134(3):653–657. doi: 10.3181/00379727-134-34854. [DOI] [PubMed] [Google Scholar]
- Dresser D. W., Wortis D. H. Use of an antiglobulin serum to detect cells producing antibody with low haemolytic efficiency. Nature. 1965 Nov 27;208(5013):859–861. doi: 10.1038/208859a0. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN H. DISTRIBUTION OF ANTIBODY PLAQUE FORMING CELLS IN VARIOUS TISSUES OF SEVERAL STRAINS OF MICE INJECTED WITH SHEEP ERYTHROCYTES. Proc Soc Exp Biol Med. 1964 Nov;117:526–530. doi: 10.3181/00379727-117-29628. [DOI] [PubMed] [Google Scholar]
- Frey-Wettstein M., Hays E. F. Immune response in preleukemic mice. Infect Immun. 1970 Oct;2(4):398–403. doi: 10.1128/iai.2.4.398-403.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H., Ceglowski W. S. Defect in cellular immunity of leukemia virus-infected mice assessed by a macrophage migration-inhibition assay. Proc Soc Exp Biol Med. 1971 Jan;136(1):154–158. doi: 10.3181/00379727-136-35216. [DOI] [PubMed] [Google Scholar]
- GROSS L. "Spontaneous" leukemia developing in C3H mice following inoculation in infancy, with AK-leukemic extracts, or AK-embrvos. Proc Soc Exp Biol Med. 1951 Jan;76(1):27–32. [PubMed] [Google Scholar]
- Hays E. F. Antigenic properties of mouse lymphoma cells. J Immunol. 1968 Feb;100(2):245–251. [PubMed] [Google Scholar]
- Hirano S., Friedman H., Ceglowski W. S. Immunosuppression by leukemia viruses. VII. Stimulatory effects of Friend leukemia virus on pre-existing antibody-forming cells to sheep erythrocytes and Escherichia coli in non-immunized mice. J Immunol. 1971 Nov;107(5):1400–1409. [PubMed] [Google Scholar]
- Häyry P., Rago D., Defendi V. Inhibition of phytohemagglutinin- and alloantigen-induced lymphocyte stimulation by Rauscher leukemia virus. J Natl Cancer Inst. 1970 Jun;44(6):1311–1319. [PubMed] [Google Scholar]
- Keast D., Stanley N. F. The induction of murine neoplasia. Pathology. 1969 Jan;1(1):19–26. doi: 10.3109/00313026909061031. [DOI] [PubMed] [Google Scholar]
- MAKINODAN T., PETERSON W. J. GROWTH AND SENESCENCE OF THE PRIMARY ANTIBODT-FORMING POTENTIAL OF THE SPLEEN. J Immunol. 1964 Dec;93:886–896. [PubMed] [Google Scholar]
- METCALF D. Reticular tumours in mice subjected to prolonged antigenic stimulation. Br J Cancer. 1961 Dec;15:769–779. doi: 10.1038/bjc.1961.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Moulds R. Immune responses in preleukaemic and leukaemic AKR mice. Int J Cancer. 1967 Jan 15;2(1):53–58. doi: 10.1002/ijc.2910020109. [DOI] [PubMed] [Google Scholar]
- Rossi G. B., Friend C. Lymphomas in mice: failure of induction after a graft-versus-host reaction. Science. 1970 Mar 6;167(3923):1383–1385. doi: 10.1126/science.167.3923.1383. [DOI] [PubMed] [Google Scholar]
- Siegel B. V., Neher G. H., Morton J. I. Quantitation and ultrastructure of immunodepression in murine virus-induced leukemia. Lab Invest. 1969 Apr;20(4):347–352. [PubMed] [Google Scholar]
- Sterzl J., Ríha I. Detection of cells producing 7S antibodies by the plaque technique. Nature. 1965 Nov 27;208(5013):858–859. doi: 10.1038/208858a0. [DOI] [PubMed] [Google Scholar]
- Willems F. T., Melnick J. L., Rawls W. E. Viral inhibition of the phytohemagglutinin response of human lymphocytes and application to viral hepatitis. Proc Soc Exp Biol Med. 1969 Feb;130(2):652–661. doi: 10.3181/00379727-130-33628. [DOI] [PubMed] [Google Scholar]


