Abstract
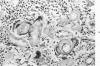
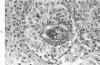
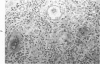
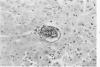
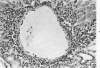
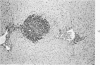
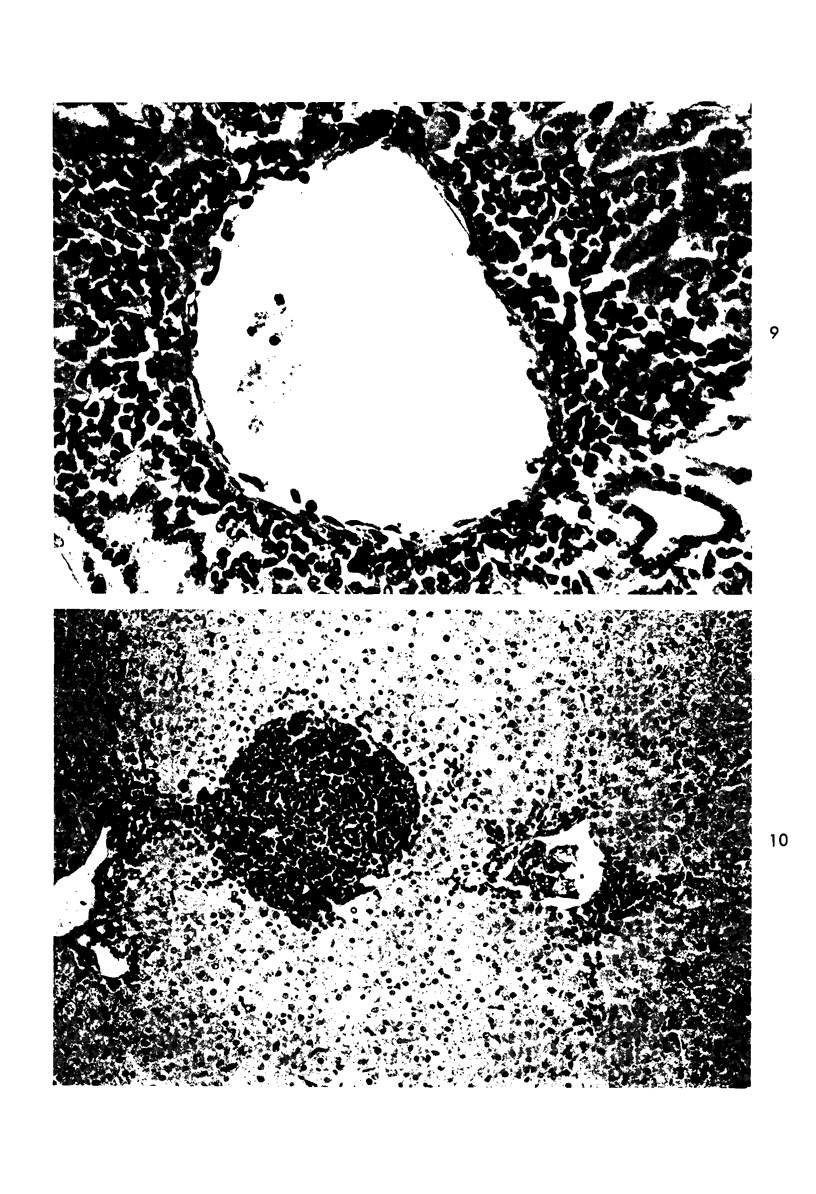
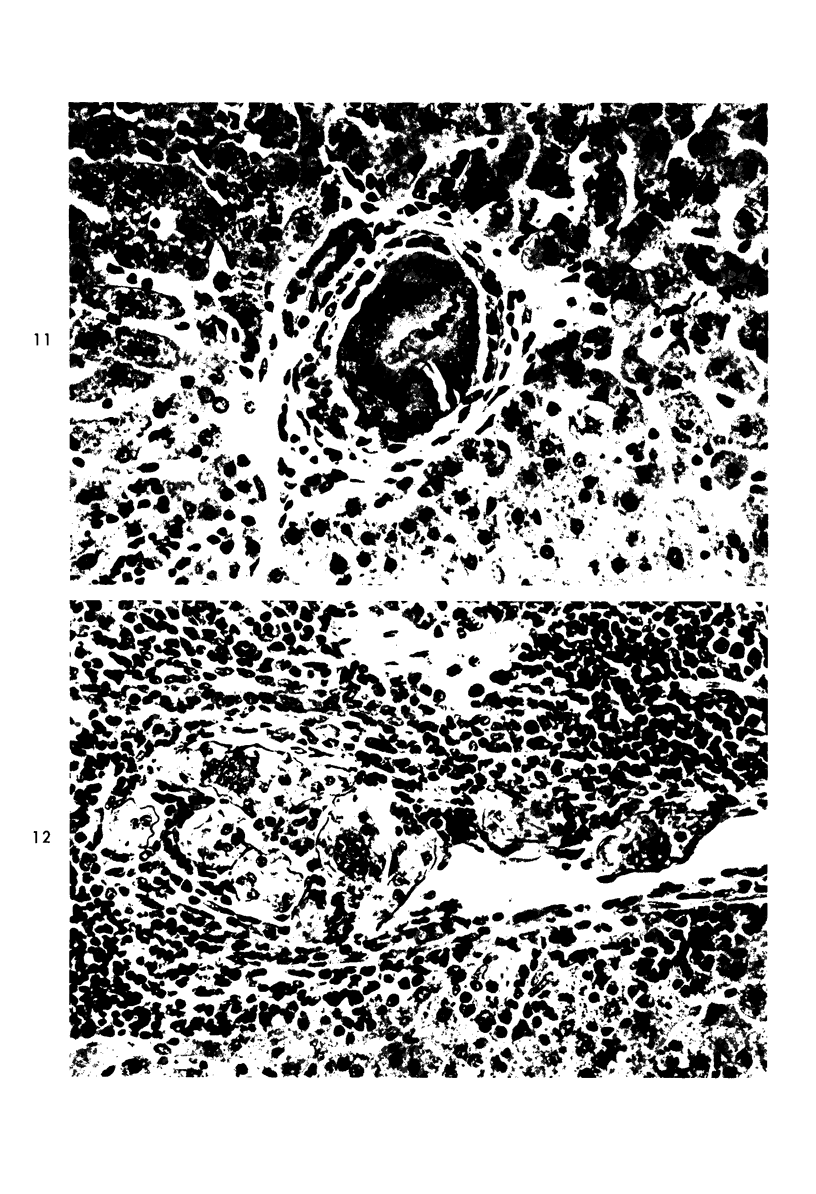
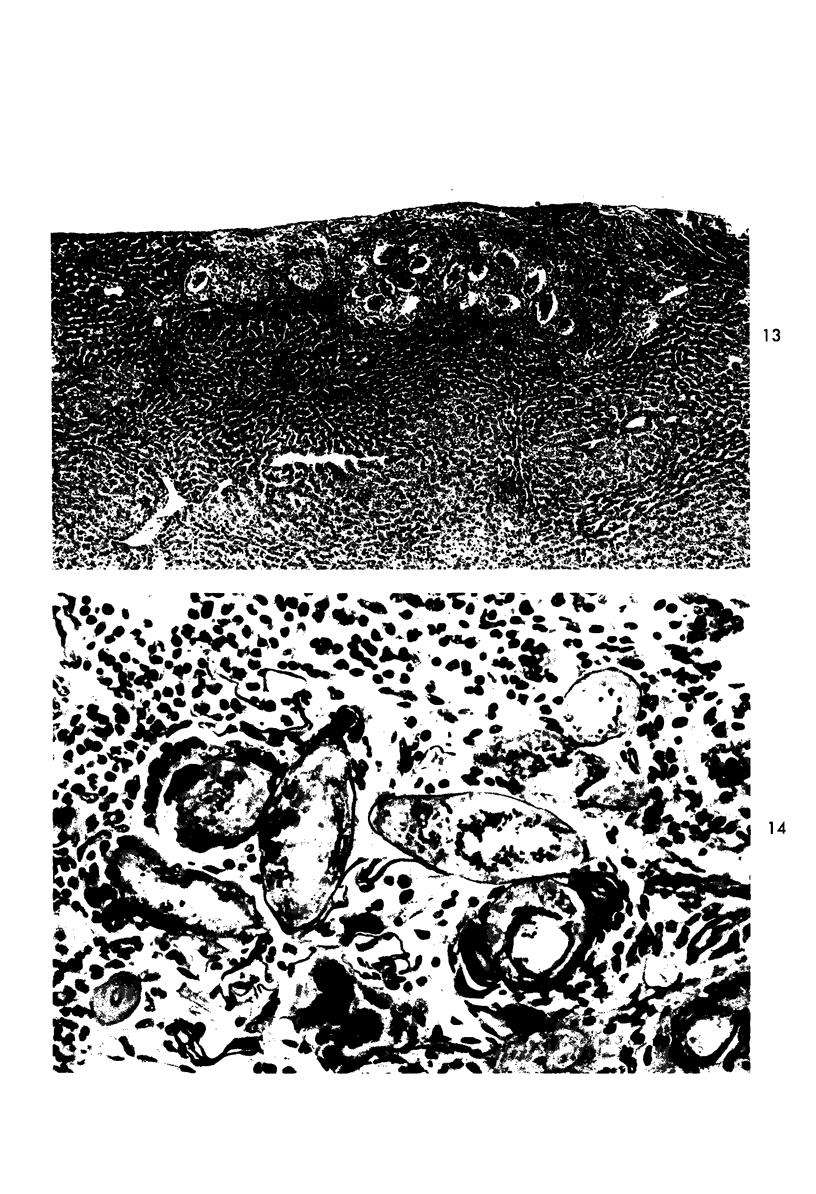
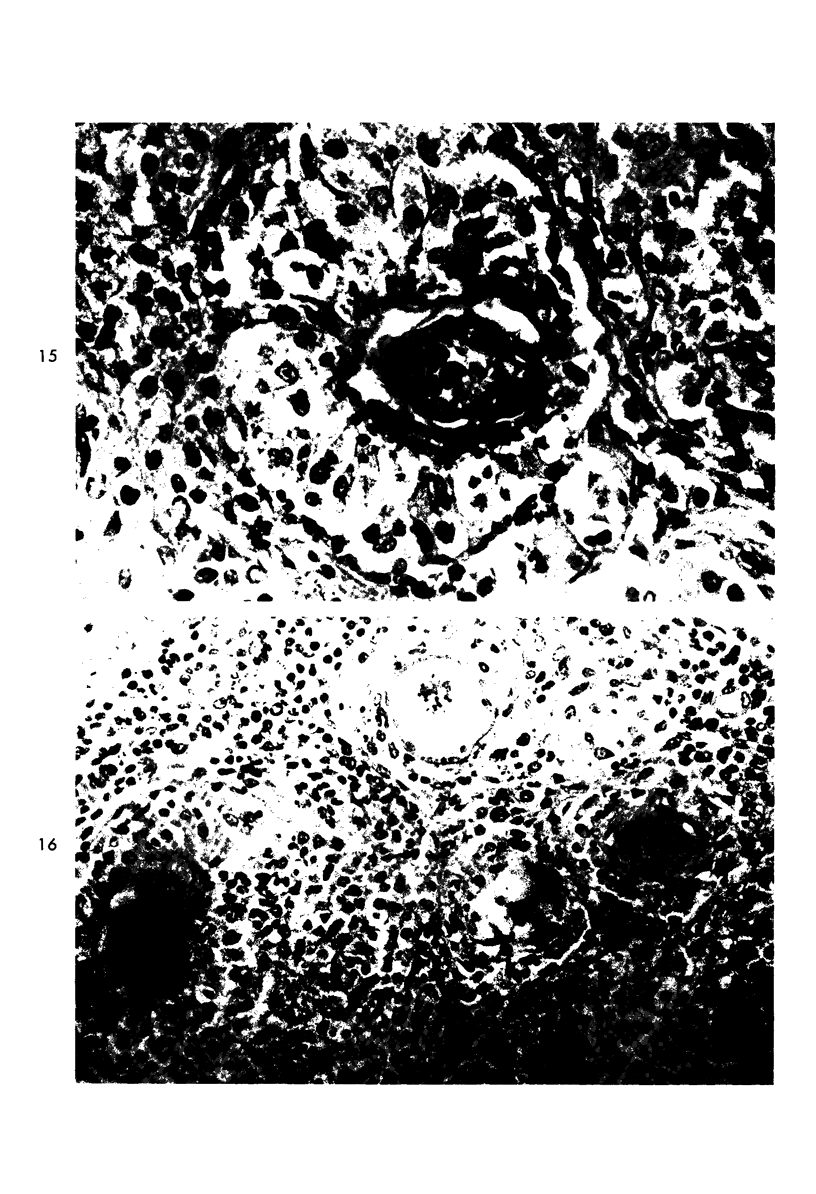
When uniform histologic criteria are applied to staging schistosome egg and granuloma development in the hamster liver, the evolution of the egg foci is shown to be monophasic, albeit with considerable variation of the individual cell response. Both real and artifactual egg-granuloma asynchrony are demonstrable. Alternate granuloma stages occur simultaneously within the same single organ, so that necrosis or fibrous scarring may result in some lesions but not in others. The granulomas of Schistosoma japonicum, S mansoni and S haematobium show both shared and distinctive features. Thus, oviposition is serial in S mansoni but clustered in the other two species. Neutrophils are common in S japonicum granulomas but are rare in the others. The differential features, listed in detail, will usually permit histologic identification of species during the early stages of infection; subsequently, the species-specific features and the overall intensity of host reaction tend to decline. At comparable egg loads and time spans, the liver pathology of S japonicum is the most severe. This is not related to granuloma size, but rather to more exudation and necrosis in early S japonicum granulomas, their tendency to encroach on adjacent liver tissue and to more extensive diffuse inflammatory infiltration. Hoeppli phenomena occur around S japonicum eggs both in stellate form, and as intraovular “reverse” precipitates. Plasma cells and amyloid deposition are frequent. Conversely, S haematobium lesions are less destructive than those of S mansoni. These findings can be correlated, to some extent, with current knowledge of the biology of schistosomes and of the antigenic components of their eggs, but several key problems concerning the immunologic host response remain to be solved.
Full text
PDF

















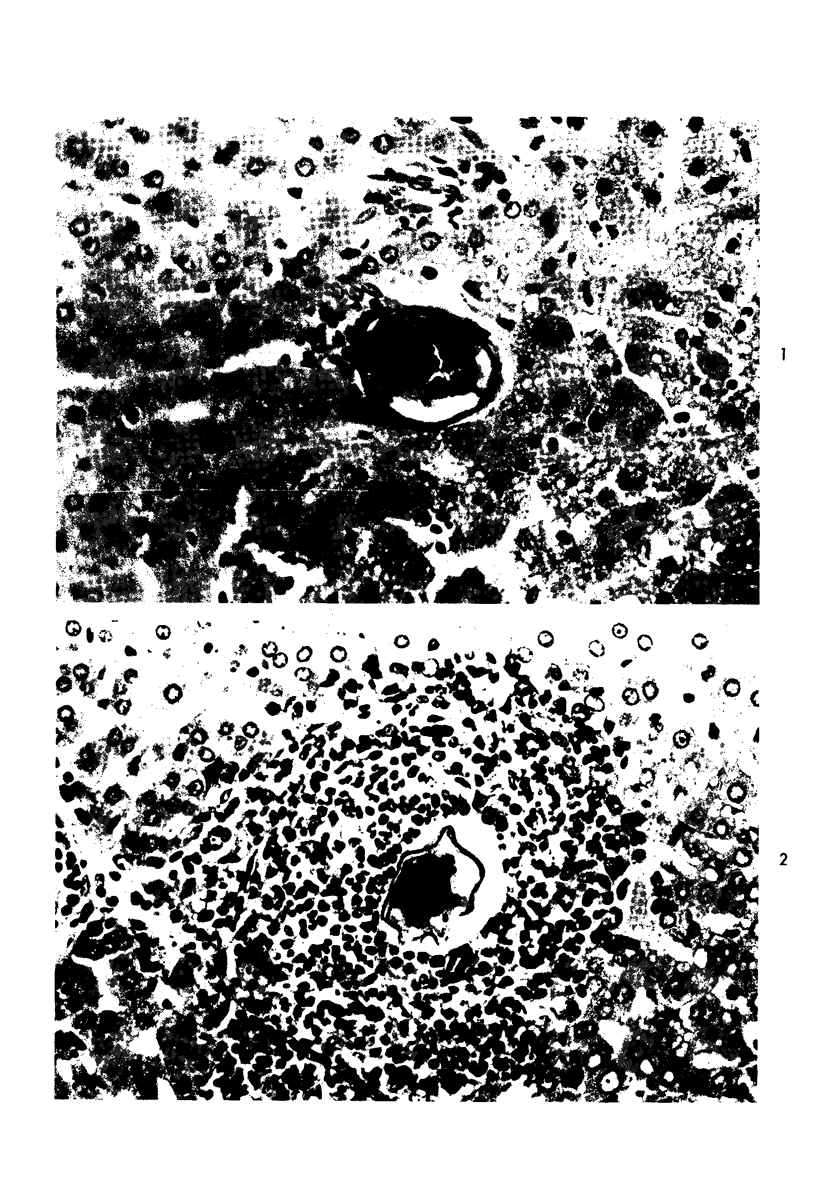
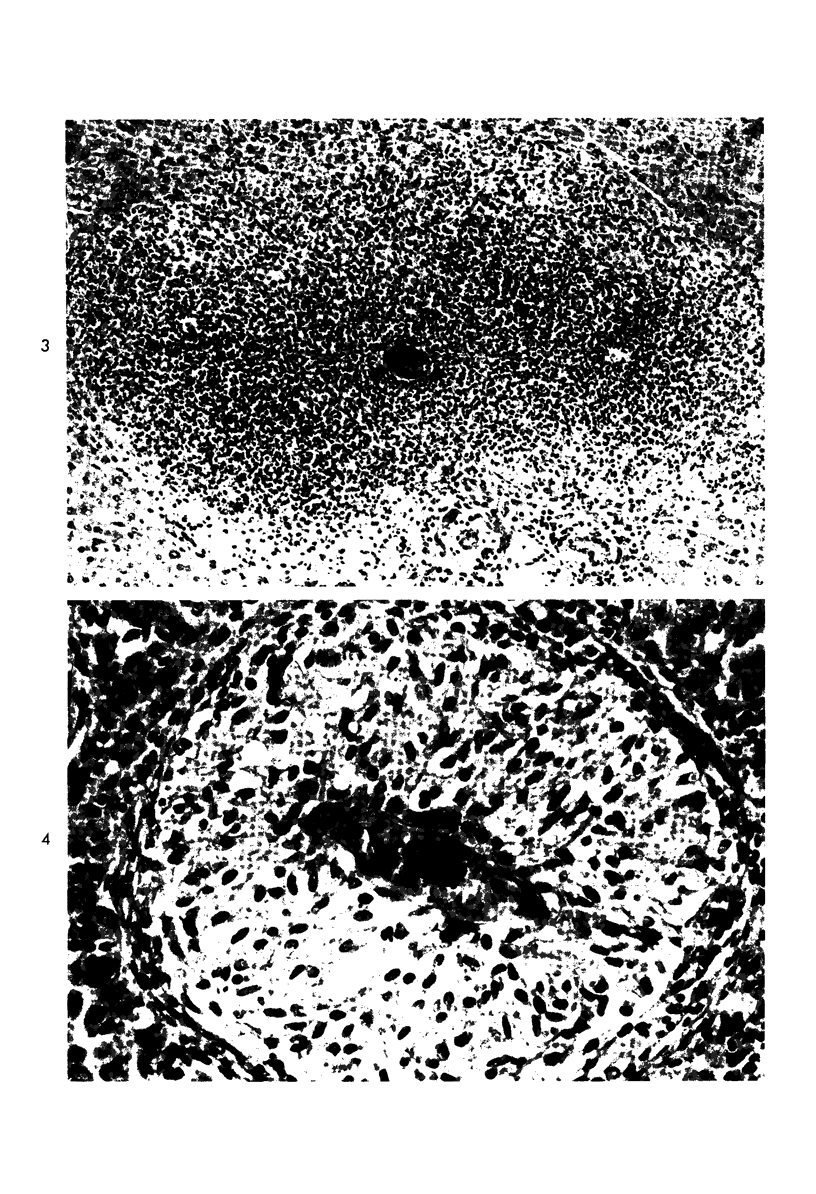
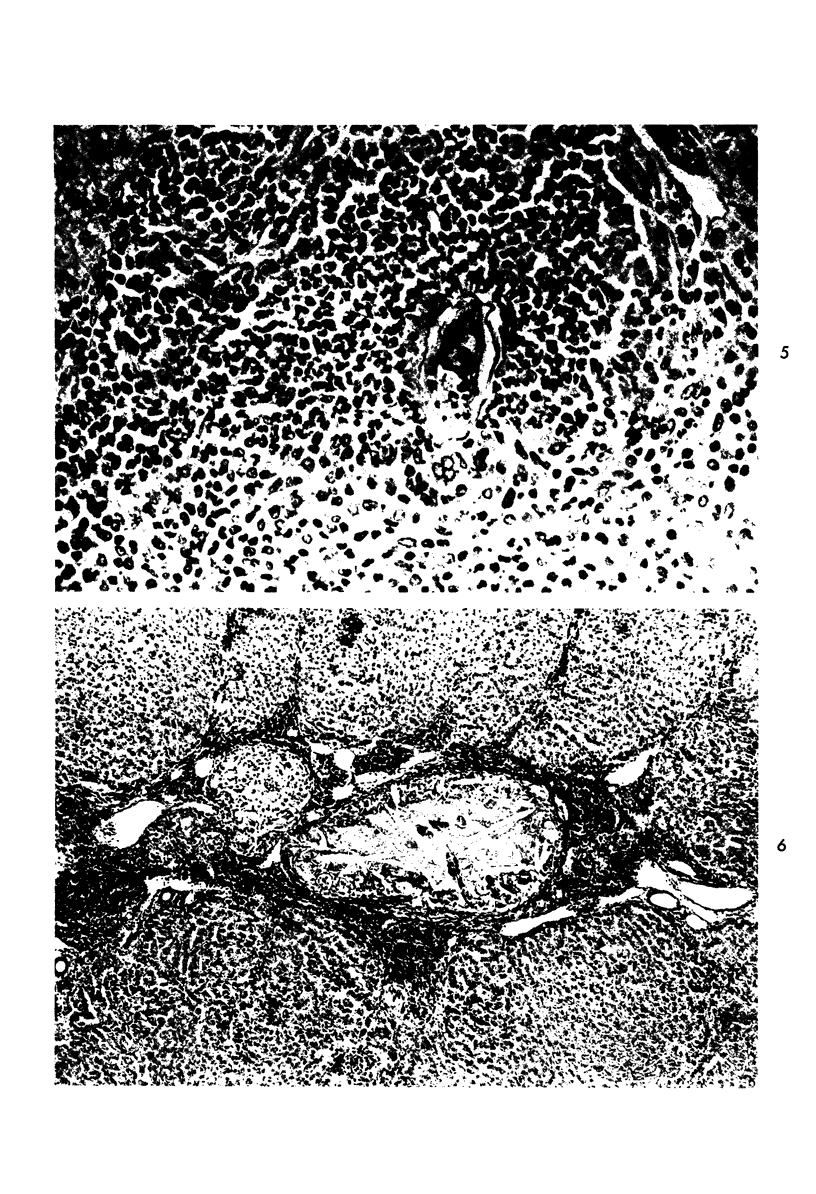
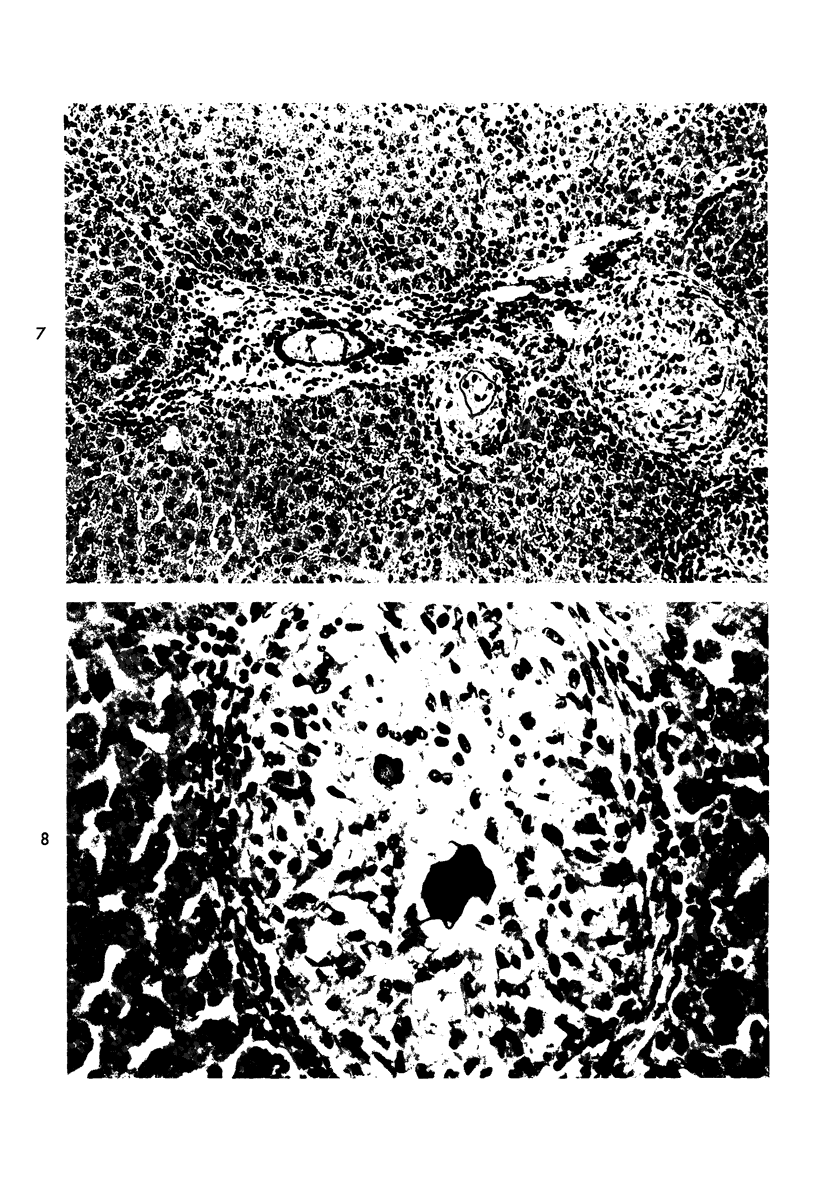
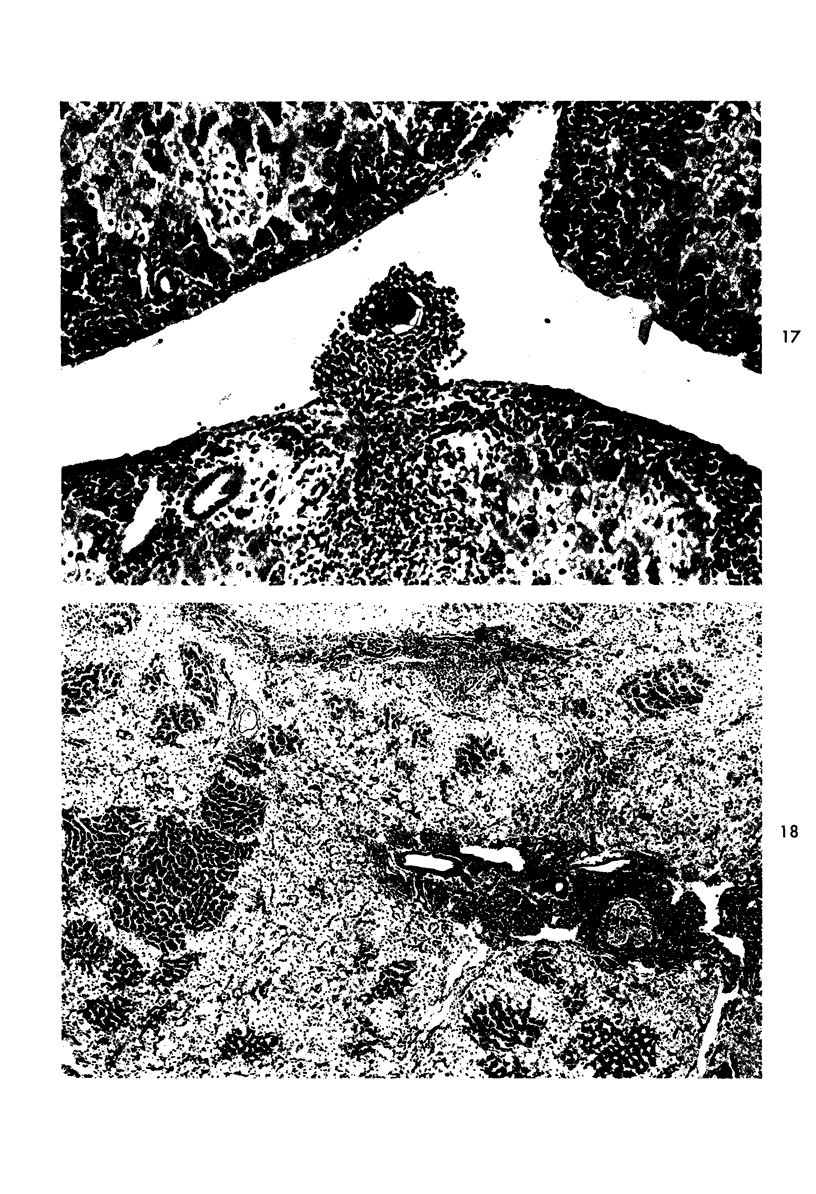
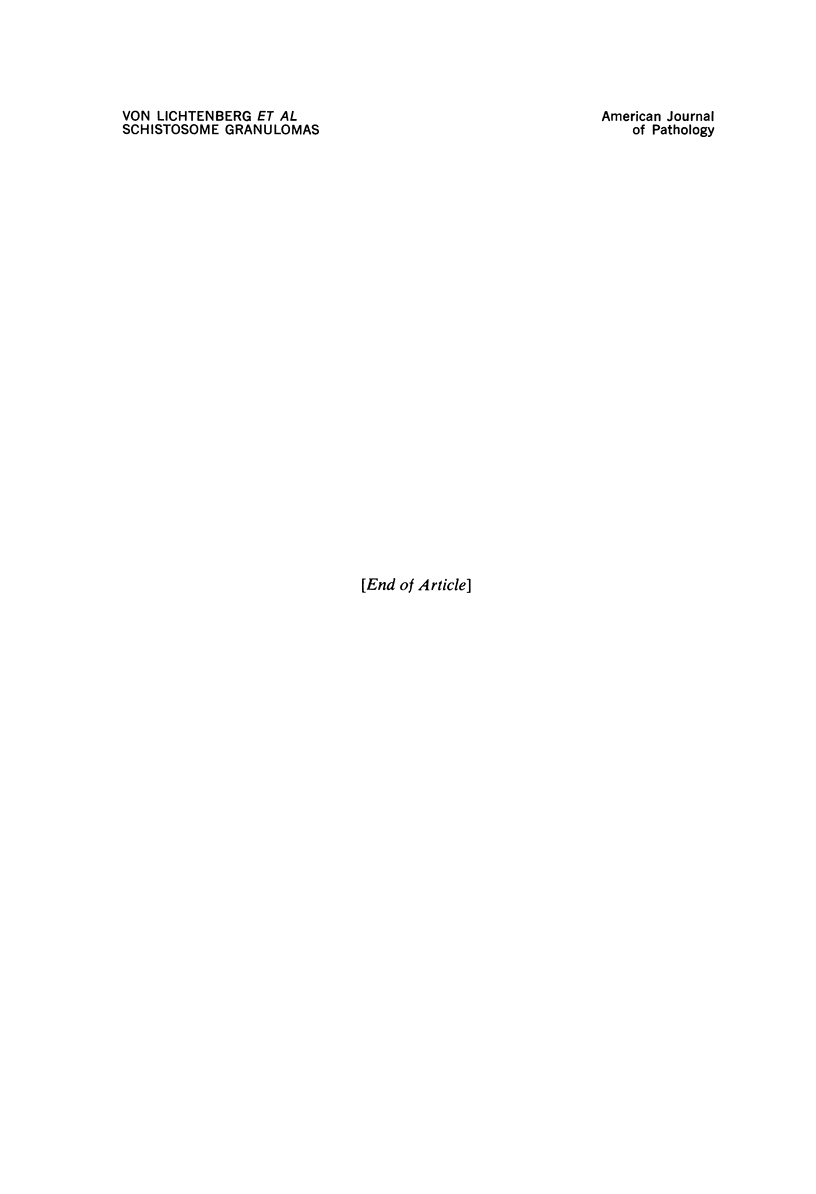
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akpom C. A., Abdel-Wahab M. F., Warren K. S. Comparison of formation of granulomata around eggs of Schistosoma mansoni in the mouse, guinea pig, rat, and hamster. Am J Trop Med Hyg. 1970 Nov;19(6):996–1000. doi: 10.4269/ajtmh.1970.19.996. [DOI] [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Specific granulomatous hypersensitivity elicited by bentonite particles coated with soluble antigens from schistosome eggs and turcle bacilli. Nature. 1971 Jan 15;229(5281):200–201. doi: 10.1038/229200a0. [DOI] [PubMed] [Google Scholar]
- CHEEVER A. W. A COMPARATIVE STUDY OF SCHISTOSOMA MANSONI INFECTIONS IN MICE, GERBILS, MULTIMAMMATE RATS AND HAMSTERS. II. QUALITATIVE PATHOLOGICAL DIFFERENCES. Am J Trop Med Hyg. 1965 Mar;14:227–238. doi: 10.4269/ajtmh.1965.14.227. [DOI] [PubMed] [Google Scholar]
- COONS A. H., LEDUC E. H., CONNOLLY J. M. Studies on antibody production. I. A method for the histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J Exp Med. 1955 Jul 1;102(1):49–60. doi: 10.1084/jem.102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever A. W. A quantitative post-mortem study of Schistosomiasis mansoni in man. Am J Trop Med Hyg. 1968 Jan;17(1):38–64. doi: 10.4269/ajtmh.1968.17.38. [DOI] [PubMed] [Google Scholar]
- Cheever A. W. Quantitative comparison of the intensity of Schistosoma mansoni infections in man and experimental animals. Trans R Soc Trop Med Hyg. 1969;63(6):781–795. doi: 10.1016/0035-9203(69)90122-9. [DOI] [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S. Endogenous desensitization: changing host granulomatou response to schistosome eggs at different stages of infection with schistosoma mansoni. Am J Pathol. 1968 Feb;52(2):369–379. [PMC free article] [PubMed] [Google Scholar]
- Edington G. M., von Lichtenberg F., Nwabuebo I., Taylor J. R., Smith J. H. Pathologic effects of schistosomiasis in Ibadan, Western State of Nigeria. I. Incidence and intensity of infection; distribution and severity of lesions. Am J Trop Med Hyg. 1970 Nov;19(6):982–995. doi: 10.4269/ajtmh.1970.19.982. [DOI] [PubMed] [Google Scholar]
- Erickson D. G., Von Lichtenberg F., Sadun E. H., Lucia H. L., Hickman R. L. Comparison of Schistosoma haematobium, S. mansoni, and S. japonicum infections in the owl monkey, Aotus trivirgatus. J Parasitol. 1971 Jun;57(3):543–558. [PubMed] [Google Scholar]
- GONNERT R. Schistosomiasis-Studien. IV. Zur Pathologie der Schistosomiasis der Maus. Z Tropenmed Parasitol. 1955 Oct;6(3):279–336. [PubMed] [Google Scholar]
- GOOD R. A., PAPERMASTER B. W. ONTOGENY AND PHYLOGENY OF ADAPTIVE IMMUNITY. Adv Immunol. 1964;27:1–115. doi: 10.1016/s0065-2776(08)60706-3. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Terry W., Harada M., Isersky C., Page D. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science. 1971 Jun 11;172(3988):1150–1151. doi: 10.1126/science.172.3988.1150. [DOI] [PubMed] [Google Scholar]
- HENTSCH H. F. Beobachtungen über die Häufigkeit kongenitaler Malaria auf Timor. Z Tropenmed Parasitol. 1955 Jun;6(2):184–187. [PubMed] [Google Scholar]
- Hsü H. F., Davis J. R., Hsü S. Y. Histopathological lesions of Rhesus monkeys and chimpanzees infected with Schistosoma japonicum. Z Tropenmed Parasitol. 1969 Jun;20(2):184–205. [PubMed] [Google Scholar]
- Kelley D. H., von Lichtenberg F. "Abnormal" schistosome oviposition. Origin of aberrant shell structures and their appearance in human tissues. Am J Pathol. 1970 Aug;60(2):271–288. [PMC free article] [PubMed] [Google Scholar]
- MELENEY H. E., SANDGROUND J. H., MOORE D. V., MOST H., CARNEY B. H. The histopathology of experimental schistosomiasis. II. Bisexual infections with S. mansoni, S. japonicum, and S. haematobium. Am J Trop Med Hyg. 1953 Sep;2(5):883–913. [PubMed] [Google Scholar]
- MOORE D. V., SANDGROUND J. H. The relative egg producing capacity of Schistosoma mansoni and Schistosoma japonicum. Am J Trop Med Hyg. 1956 Sep;5(5):831–840. doi: 10.4269/ajtmh.1956.5.831. [DOI] [PubMed] [Google Scholar]
- Moore D. E., Warren K. S. Hepatosplenic Schistosomiasis mansoni and japonica compared in mice each infected with one pair of worms. Trans R Soc Trop Med Hyg. 1967;61(1):104–109. doi: 10.1016/0035-9203(67)90059-4. [DOI] [PubMed] [Google Scholar]
- PELLEGRINO J., OLIVEIRA C. A., FARIA J., CUNHA A. S. New approach to the screening of drugs in experimental schistosomiasis mansoni in mice. Am J Trop Med Hyg. 1962 Mar;11:201–215. doi: 10.4269/ajtmh.1962.11.201. [DOI] [PubMed] [Google Scholar]
- RITCHIE L. S., LIN S., MOON A. P., FRICK L. P., WILLIAMS J. E., ASAKURA S., HISHINUMA Y. The possible effects of pH and specific gravity on the ether-sedimentation procedure in concentrating eggs and cysts. Am J Trop Med Hyg. 1960 Jul;9:444–449. doi: 10.4269/ajtmh.1960.9.444. [DOI] [PubMed] [Google Scholar]
- Sadun E. H., Von Lichtenberg F., Cheever A. W., Erickson D. G., Hickman R. L. Experimental infection with Schistosoma haematobium in chimpanzees. Am J Trop Med Hyg. 1970 May;19(3):427–458. doi: 10.4269/ajtmh.1970.19.427. [DOI] [PubMed] [Google Scholar]
- Sadun E. H., von Lichtenberg F., Cheever A. W., Erickson D. G. Schistosomiasis mansoni in the chimpanzee. The natural history of chronic infections after single and multiple exposures. Am J Trop Med Hyg. 1970 Mar;19(2):258–277. [PubMed] [Google Scholar]
- Smith J. H., Von Lichtenberg F. The Hoeppli phenomenon in schistosomiasis. II. Histochemistry. Am J Pathol. 1967 Jun;50(6):993–1007. [PMC free article] [PubMed] [Google Scholar]
- Smith T. M., Lucia H. L., Doughty B. L., von Lichtenberg F. C. The role of phospholipids in schistosome granulomas. J Infect Dis. 1971 Jun;123(6):629–639. doi: 10.1093/infdis/123.6.629. [DOI] [PubMed] [Google Scholar]
- VON LICHTENBERG STUDIES ON GRANULOMA FORMATION. III. ANTIGEN SEQUESTRATION AND DESTRUCTION IN THE SCHISTOSOME PSEUDOTUBERCLE. Am J Pathol. 1964 Jul;45:75–94. [PMC free article] [PubMed] [Google Scholar]
- Von Lichtenberg F., Edington G. M., Nwabuebo I., Taylor J. R., Smith J. H. Pathologic effects of schistomiasis in Ibadan Western State of Nigeria. II. Pathogenesis of lesions of the bladder and ureters. Am J Trop Med Hyg. 1971 Mar;20(2):244–254. doi: 10.4269/ajtmh.1971.20.244. [DOI] [PubMed] [Google Scholar]
- Von Lichtenberg F., Sadun E. H., Cheever A. W., Erickson D. G., Johnson A. J., Boyce H. W. Experimental infection with Schistosoma japonicum in chimpanzees. Am J Trop Med Hyg. 1971 Nov;20(6):850–893. doi: 10.4269/ajtmh.1971.20.850. [DOI] [PubMed] [Google Scholar]
- Von Lichtenberg F., Smith T. M., Lucia H. L., Doughty B. L. New model for schistosome granuloma formation using a sluble egg antigen and bentonite particles. Nature. 1971 Jan 15;229(5281):199–200. doi: 10.1038/229199a0. [DOI] [PubMed] [Google Scholar]
- Warren K. S., Berry E. G. Induction of hepatosplenic disease by single pairs of the Philippine, Formosan, Japanese, and Chinese strains of Schistosoma japonicum. J Infect Dis. 1972 Nov;126(5):482–491. doi: 10.1093/infdis/126.5.482. [DOI] [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O., Cowan R. B. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am J Pathol. 1967 Nov;51(5):735–756. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O. Granuloma formation around Schistosoma mansoni, S. HAEMATOBIUM, AND S. japonicum eggs. Size and rate of development, cellular composition, cross-sensitivity, and rate of egg destruction. Am J Trop Med Hyg. 1970 Mar;19(2):292–304. doi: 10.4269/ajtmh.1970.19.292. [DOI] [PubMed] [Google Scholar]
- Warren K. S. Intestinal ostruction in murine schistosomiasis japonica. Gastroenterology. 1969 Dec;57(6):697–702. [PubMed] [Google Scholar]
- von LICHTENBERG Host response to eggs of S. mansoni. I. Granuloma formation in the unsensitized laboratory mouse. Am J Pathol. 1962 Dec;41:711–731. [PMC free article] [PubMed] [Google Scholar]
- von Lichtenberg F., Smith J. H., Cheever A. W. The Hoeppli phenomenon in schistosomiasis. Comparative pathology and immunopathology. Am J Trop Med Hyg. 1966 Nov;15(6):886–895. doi: 10.4269/ajtmh.1966.15.886. [DOI] [PubMed] [Google Scholar]