Abstract
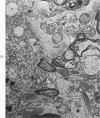
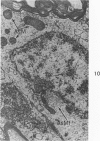
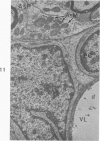
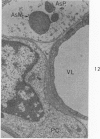
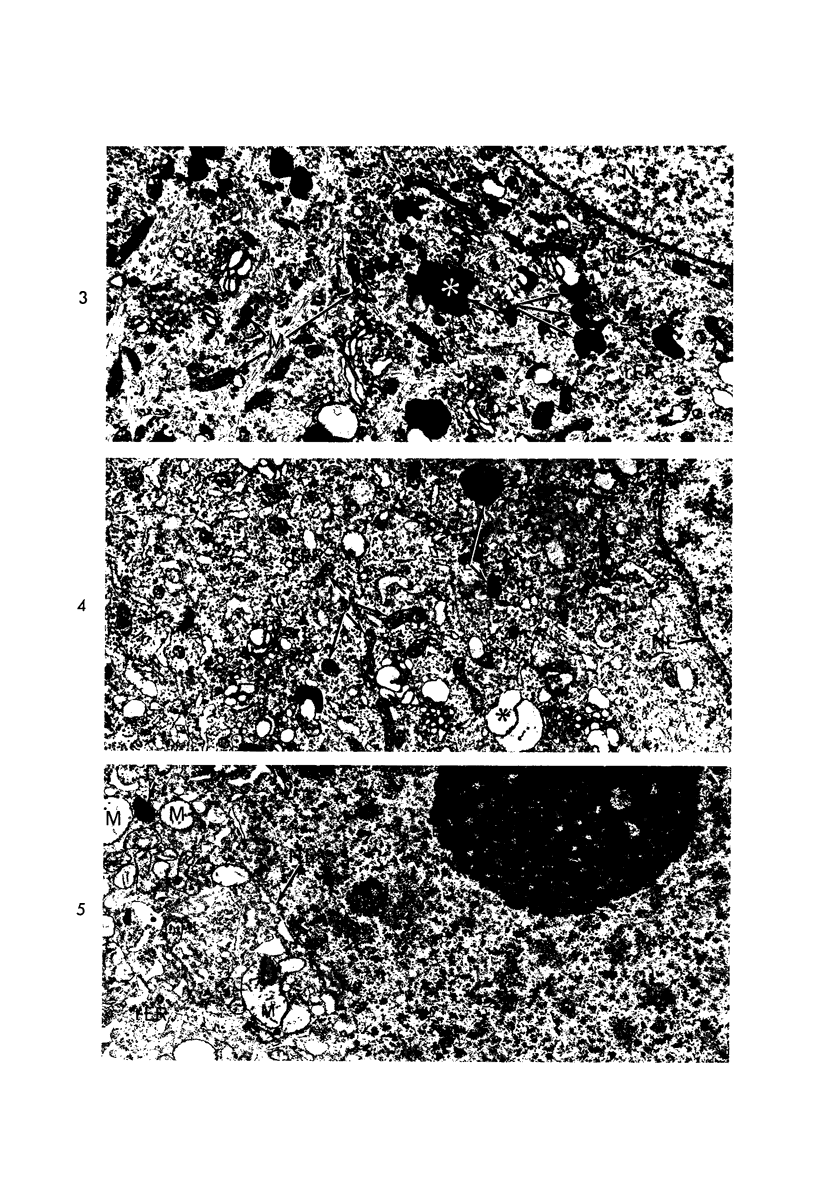
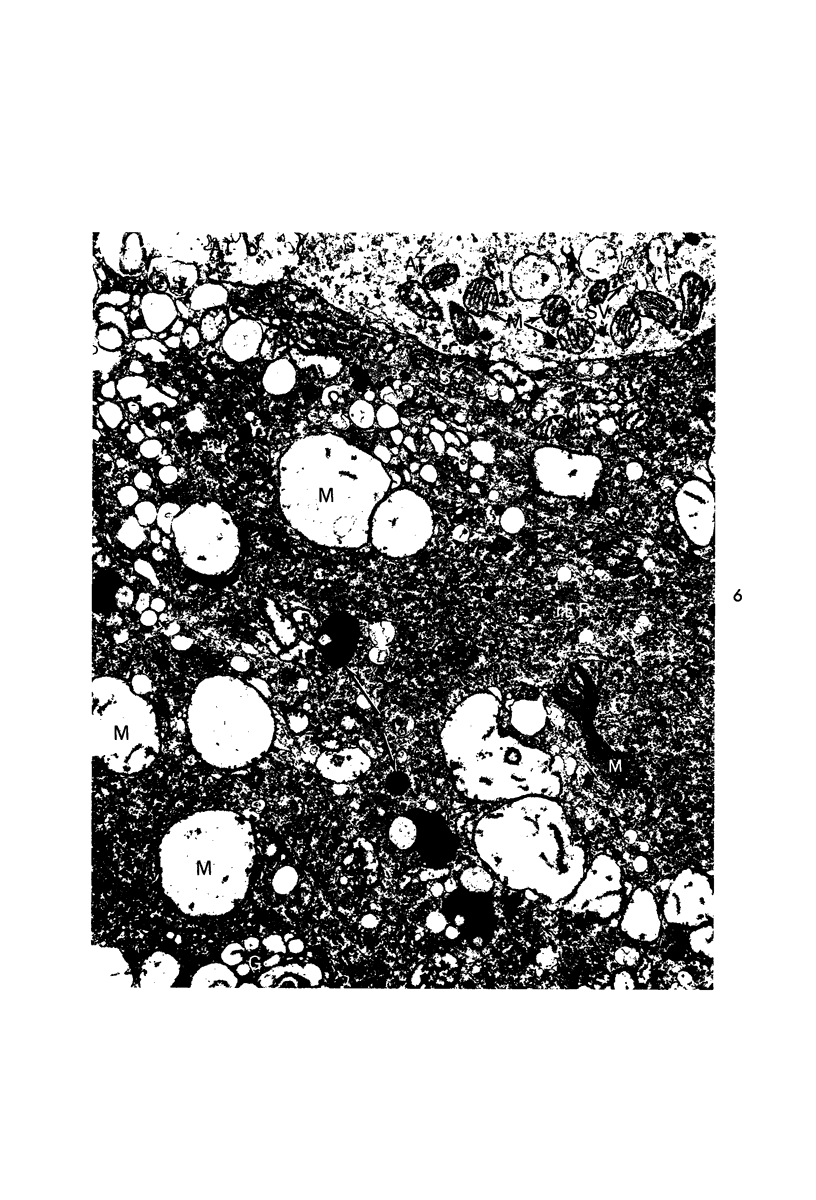
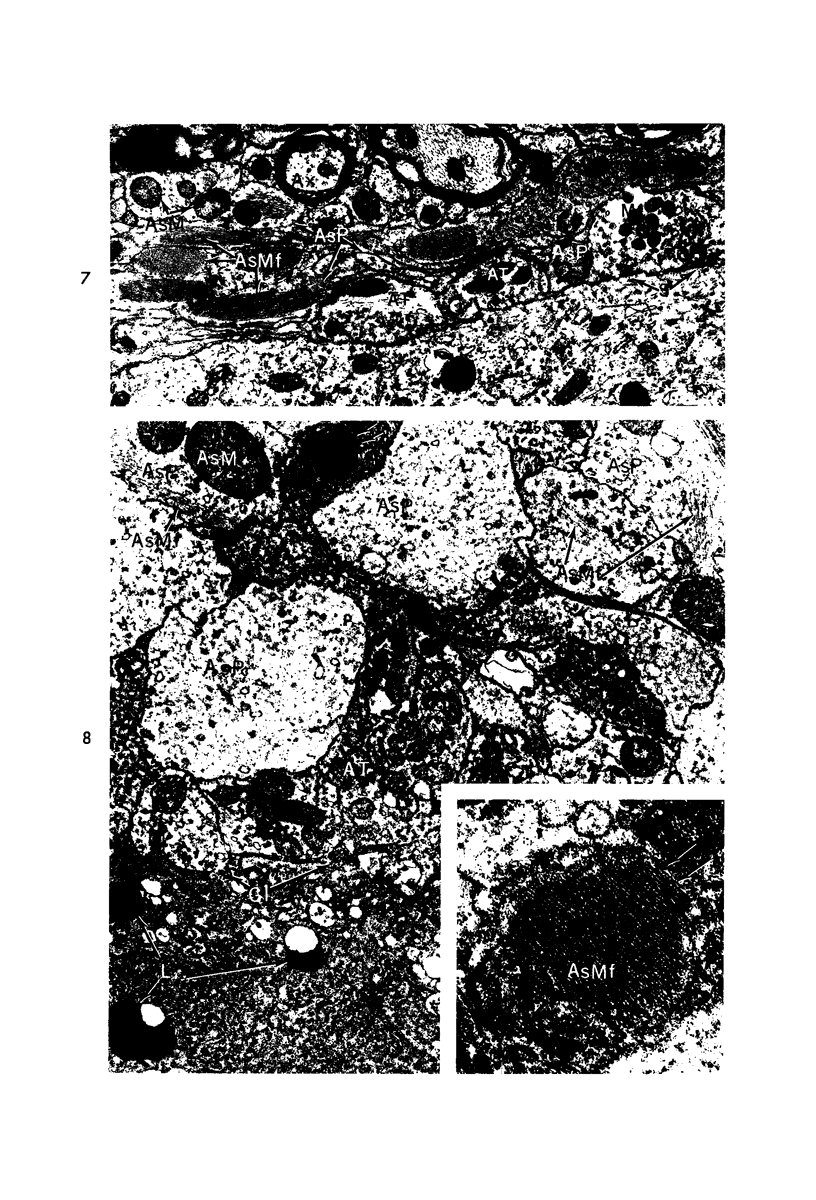
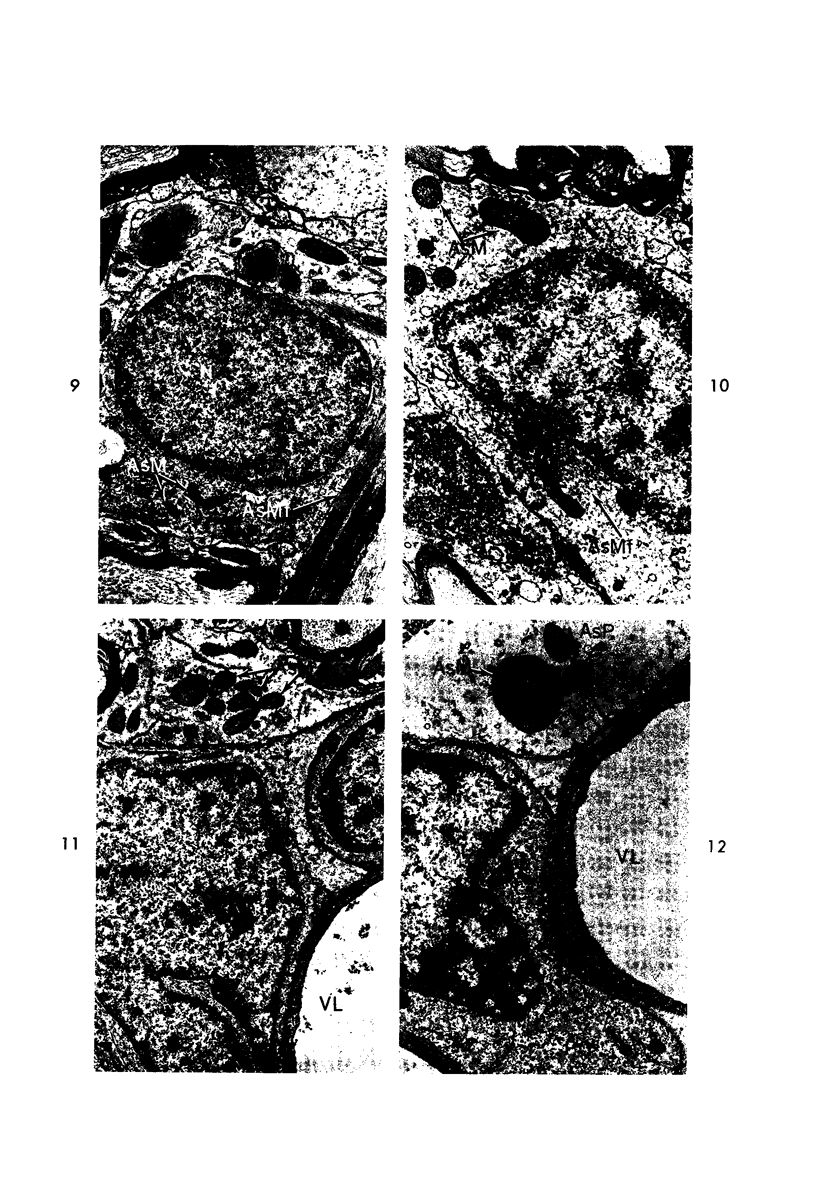
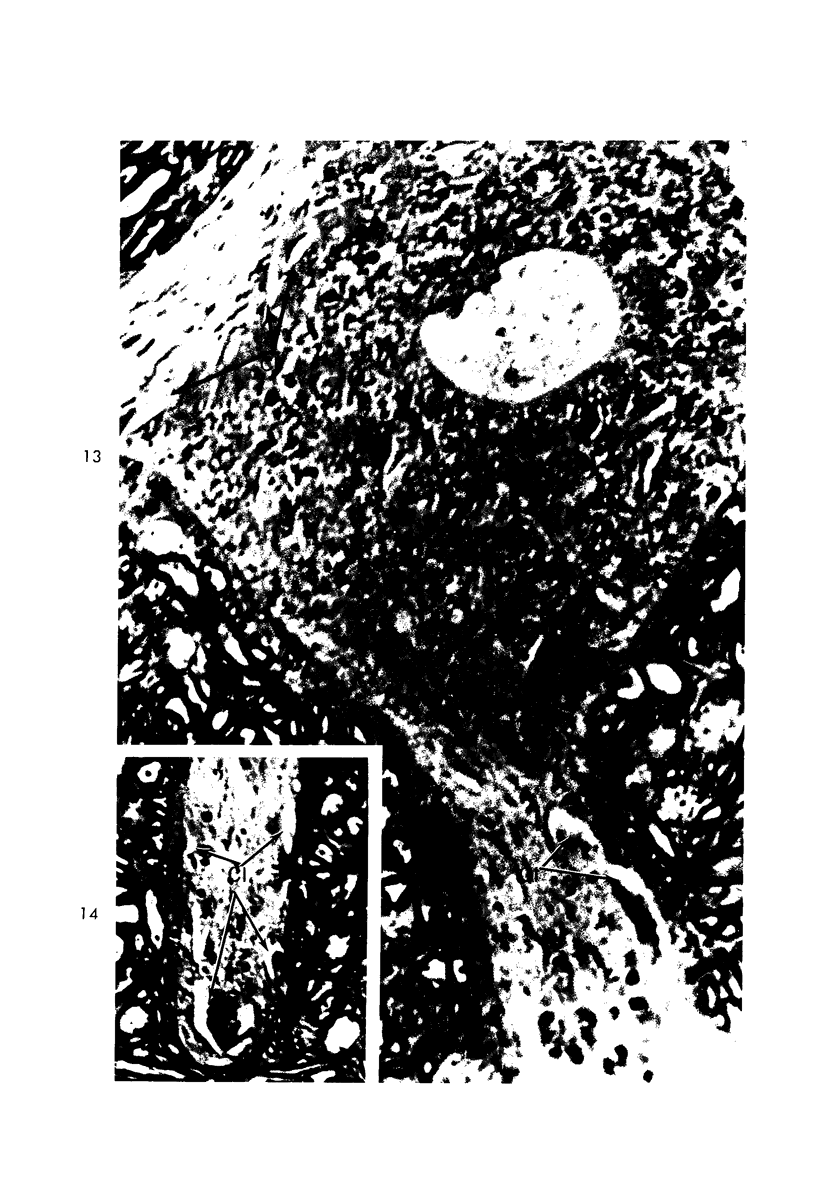
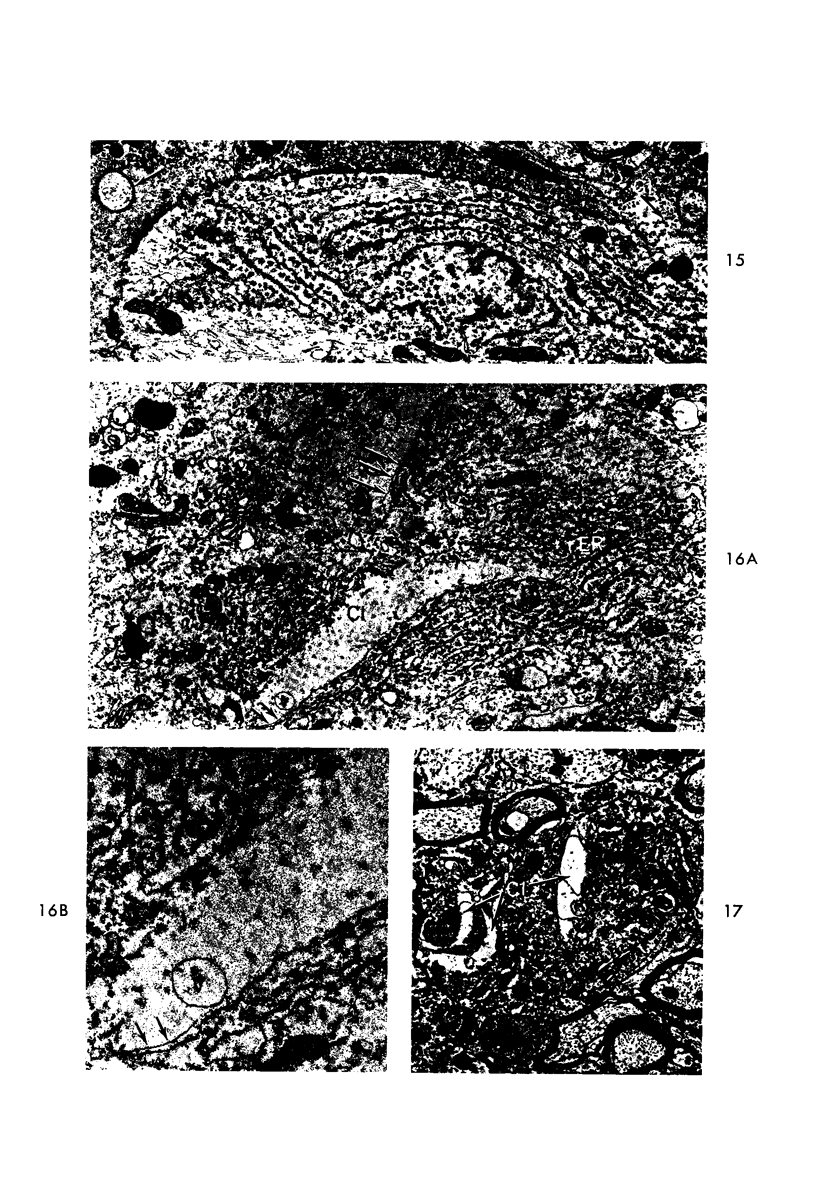
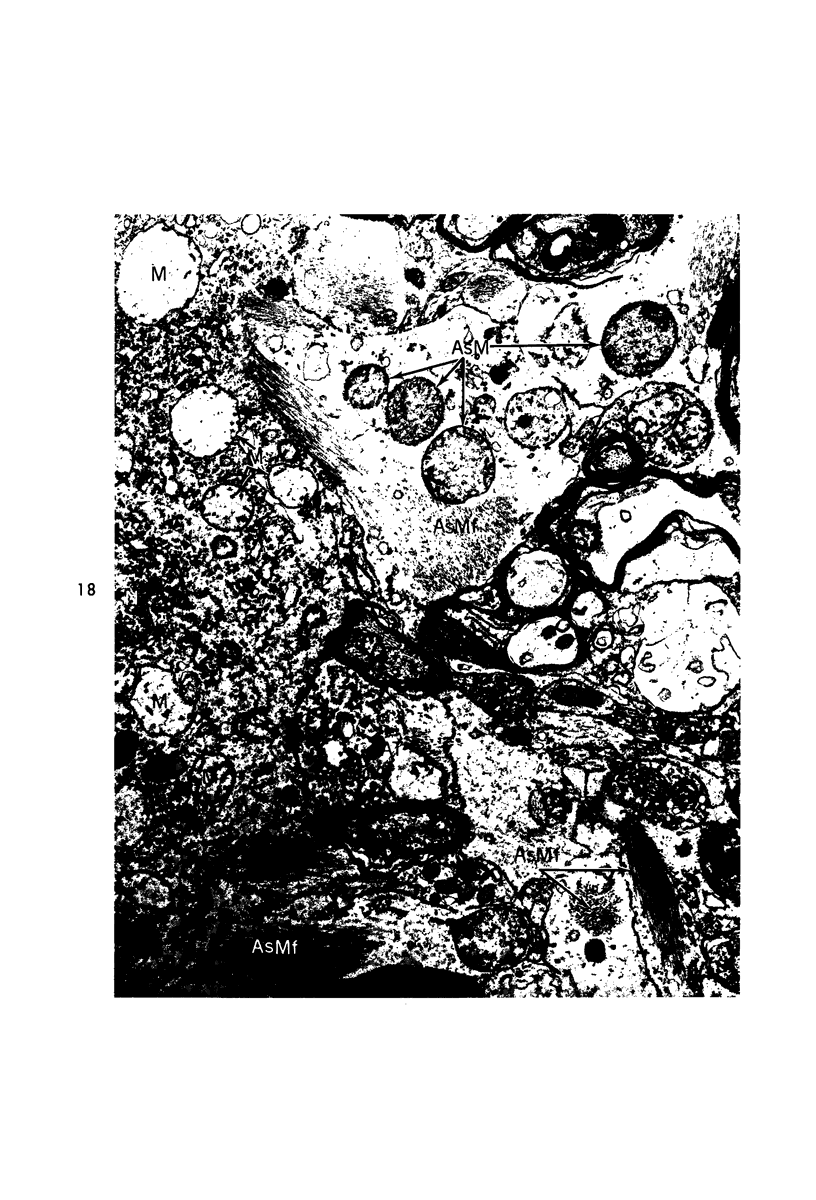
Circulatory arrest to the lumbar spinal cord of adult cat was produced by occlusion of the descending aorta and concurrent arterial hypotension. Local hypothermia of the cord was induced by paraffin oil at 5 C, which was circulated over the exposed surface of the cord, using the laminectomy wound as a trough. Intramedullary temperature was 15 C at a depth of 5 mm. In 10 control animals oil at 37 or 5 C was circulated over the exposed cords (normal-normothermic and normal-hypothermic controls with 1 and 2 hours hypothermia). Three animals had circulatory arrest and recirculation in normothermia (ischemic-normothermic) and 3 in hypothermia (ischemic-simultaneous hypothermia). Three had circulatory arrest and 15 minutes of recirculation in normothermia followed by 1 hour of hypothermia (ischemic-delayed hypothermia). The medial and lateral portions of the anterior gray horns of the last lumbar spinal segment were studied in the light and electron microscopes. Ischemic-normothermic tissue showed 20% shrinkage in mean areas of neuronal perikarya and massive “watery” swelling of astrocytic cell bodies and processes. Within neuronal perikarya and dendrites, cytoplasm increased in electron density, ribosomes dispersed, Golgi apparatus swelled and mitochondria swelled with loss of matrix density and disruption of cristae. Axons and axon terminals did not increase in size, but mitochondria within these structures doubled in size without loss of matrix density or change in pattern of cristae. Synaptic vesicles were no longer uniform in size, and they were clumped away from the synaptic cleft and diminished in number. Lysosomes were unchanged in appearance and size. Mitochondria of astrocytes underwent approximately fourfold enlargement without loss of matrix density or pattern of cristae. Bundles of astrocytic microfilaments were fragmented, spread apart and diminished in quantity. Oligodendroglia and endothelial cells were unchanged. Normal-hypothermic animals were similar to normal-normothermic except for clefts in rough endoplasmic reticulum of neurons and dendrites. These clefts were formed by a separation of the cisternal membrane from the adjacent row of ribosomal rosettes. Ischemic-simultaneous hypothermia animals had findings identical to normal-hypothermic animals. Ischemic-delayed hypothermia animals were similar to ischemic-normothermic animals except for less swelling of astrocytic processes, greater swelling of astrocytic mitochondria and less alteration of microfilaments. The findings show that ischemia in normothermia brings about alterations in virtually every organelle of the neurronal perikaryon except the lysosome. Simultaneous hypothermia in ischemia prevents the protean alterations of ischemia, whereas hypothermia delayed until after the ischemic episode only slightly modifies the cellular lesions found in ischemic-normothermic animals.
Full text
PDF



















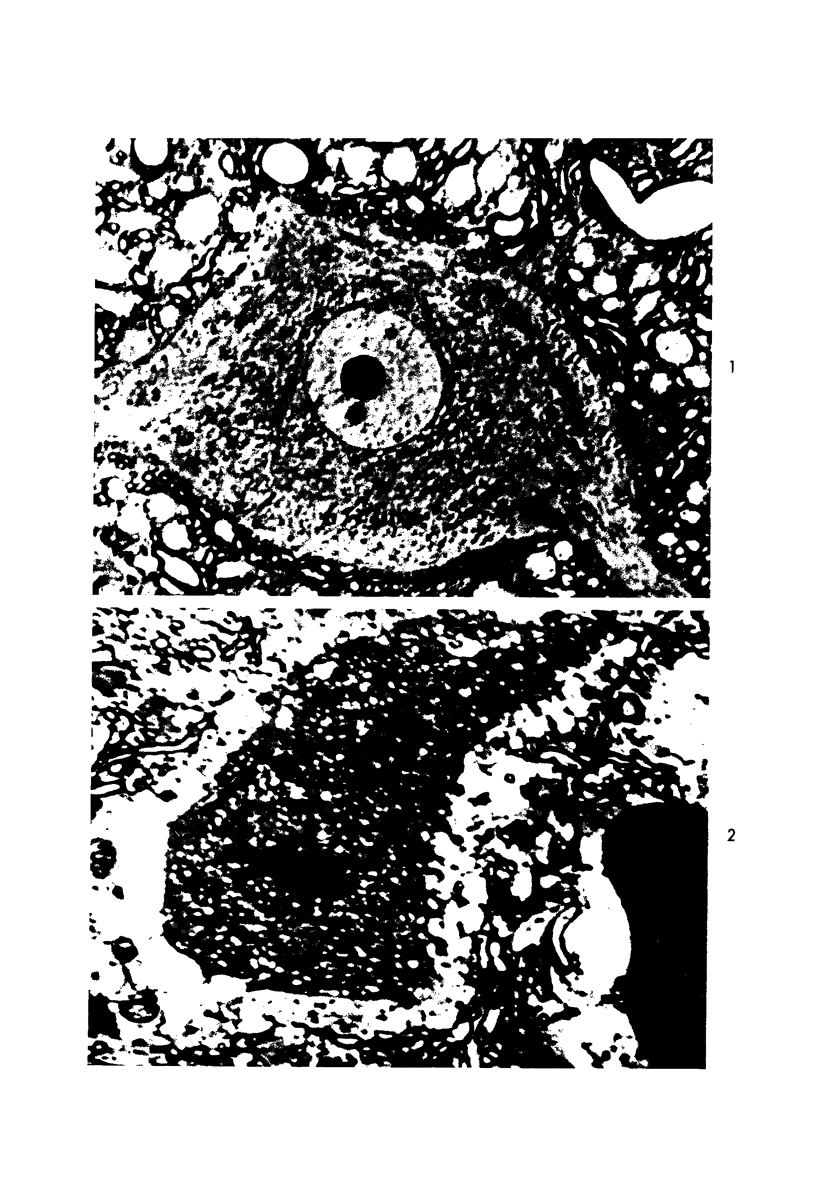
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albin M. S., White R. J., Acosta-Rua G., Yashon D. Study of functional recovery produced by delayed localized cooling after spinal cord injury in primates. J Neurosurg. 1968 Aug;29(2):113–120. doi: 10.3171/jns.1968.29.2.0113. [DOI] [PubMed] [Google Scholar]
- BECKER N. H., BARRON K. D. The cytochemistry of anoxic and anoxic-ischemic encephalopathy in rats. I. Alterations in neuronal lysosomes identified by acid phosphatase activity. Am J Pathol. 1961 Feb;38:161–175. [PMC free article] [PubMed] [Google Scholar]
- Bakay L., Lee J. C. The effect of acute hypoxia and hypercapnia on the ultrastructure of the central nervous system. Brain. 1968;91(4):697–706. doi: 10.1093/brain/91.4.697. [DOI] [PubMed] [Google Scholar]
- Bourke R. S., Nelson K. M., Naumann R. A., Young O. M. Studies of the production and subsequent reduction swelling in primate. Cerebral cortex under isosmotic conditions in vivo. Exp Brain Res. 1970;10(4):427–446. doi: 10.1007/BF02324768. [DOI] [PubMed] [Google Scholar]
- Chiang J., Kowada M., Ames A., 3rd, Wright R. L., Majno G. Cerebral ischemia. III. Vascular changes. Am J Pathol. 1968 Feb;52(2):455–476. [PMC free article] [PubMed] [Google Scholar]
- Clendenon N. R., Allen N., Komatsu T., Liss L., Gordon W. A., Heimberger K. Biochemical alterations in the anoxic-ischemic lesion of rat brain. Arch Neurol. 1971 Nov;25(5):432–448. doi: 10.1001/archneur.1971.00490050066006. [DOI] [PubMed] [Google Scholar]
- Echandia E. L., Piezzi R. S. Microtubules in the nerve fibers of the toad Bufo arenarum Hensel. Effect of low temperature on the sciatic nerve. J Cell Biol. 1968 Nov;39(2):491–497. doi: 10.1083/jcb.39.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELFAN S., TARLOV I. M. ALTERED NEURON POPULATION IN L7 SEGMENT OF DOGS WITH EXPERIMENTAL HIND-LIMB RIGIDITY. Am J Physiol. 1963 Sep;205:606–616. doi: 10.1152/ajplegacy.1963.205.3.606. [DOI] [PubMed] [Google Scholar]
- GELFAN S., TARLOV I. M. Interneurones and rigidity of spinal origin. J Physiol. 1959 Jun 11;146(3):594–617. doi: 10.1113/jphysiol.1959.sp006214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambetti P., Gonatas N. K., Flexner L. B. The fine structure of puromycin-induced changes in mouse entorhinal cortex. J Cell Biol. 1968 Feb;36(2):379–390. doi: 10.1083/jcb.36.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. H., Cox J. V., Hudgins W. R. Ultrastructure of the microvasculature in experimental cerebral infarction. Acta Neuropathol. 1971;18(4):273–285. doi: 10.1007/BF00688441. [DOI] [PubMed] [Google Scholar]
- Klatzo I., Li C. L., Long D. M., Bak A. F., Mossakowski M. J., Parker L. O., Rasmussen L. E. The effect of hypothmia on electric impedance and penetration of substances from the CSF into the periventricular brain tissue. Prog Brain Res. 1968;29:385–399. doi: 10.1016/s0079-6123(08)64170-9. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- McGee-Russell S. M., Brown A. W., Brierley J. B. A combined light and electron microscope study of early anoxic-ischaemic cell change in rat brain. Brain Res. 1970 Jun 3;20(2):193–200. doi: 10.1016/0006-8993(70)90288-x. [DOI] [PubMed] [Google Scholar]
- Olsson Y., Hossmann K. A. The effect of intravascular saline perfusion on the sequelae of transient cerebral ischemia. Light and electron microscopial observations. Acta Neuropathol. 1971;17(1):68–79. doi: 10.1007/BF00684742. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Seta K., Takeda H., Ando K., Handa H., Araki C. On the isolation of mitochondria with high respiratory control from rat brain. J Biochem. 1966 May;59(5):501–510. doi: 10.1093/oxfordjournals.jbchem.a128334. [DOI] [PubMed] [Google Scholar]
- Pásztor E., Hámori J. Effect of local cooling on the fine structure of the cerebral and cerebellar cortex. Acta Morphol Acad Sci Hung. 1971;19(1):81–87. [PubMed] [Google Scholar]
- ROSOMOFF H. L., GILBERT R. Brain volume and cerebrospinal fluid pressure during hypothermia. Am J Physiol. 1955 Oct;183(1):19–22. doi: 10.1152/ajplegacy.1955.183.1.19. [DOI] [PubMed] [Google Scholar]
- Roth L. E. Electron microscopy of mitosis in amebae. 3. Cold and urea treatments: a basis for tests of direct effects of mitotic inhibitors on microtubule formation. J Cell Biol. 1967 Jul;34(1):47–59. doi: 10.1083/jcb.34.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. L., Case N. M. A modified aldehyde perfusion technique for preventing certain artifacts in electron microscopy of the central nervous system. J Microsc. 1970;92(2):69–84. doi: 10.1111/j.1365-2818.1970.tb02240.x. [DOI] [PubMed] [Google Scholar]
- Schutz H., Silverstein P. R., Vapalahti M., Bruce D. A., Mela L. M., Miller L. D., Langfitt T. W. Function of brain mitochondria after increased intracranial pressure. Surg Forum. 1972;23(0):411–413. [PubMed] [Google Scholar]
- Shay J. Morphometry of an ischemic lesion of cat spinal cord. Am J Pathol. 1973 Sep;72(3):397–402. [PMC free article] [PubMed] [Google Scholar]
- Silverstein P. R., Schutz H., Vapalahti M., Bruce D. A., Mela L. M., Miller L. D., Langfitt T. W. Function of brain mitochondria after profound hypoxemia. Surg Forum. 1972;23(0):72–74. [PubMed] [Google Scholar]
- Tilney L. G., Porter K. R. Studies on the microtubules in heliozoa. II. The effect of low temperature on these structures in the formation and maintenance of the axopodia. J Cell Biol. 1967 Jul;34(1):327–343. doi: 10.1083/jcb.34.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towfighi J., Gonatas N. K. Effect of intracerebral injection of ouabain in adult and developing rats. An ultrastructural and autoradiographic study. Lab Invest. 1973 Feb;28(2):170–180. [PubMed] [Google Scholar]
- VAN HARREVELD A., SCHADE J. P. Nerve cell destruction by asphyxiation of the spinal cord. J Neuropathol Exp Neurol. 1962 Jul;21:410–423. doi: 10.1097/00005072-196207000-00009. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A., Khattab F. I. Electron microscopy of asphyxiated spinal cords of cats. J Neuropathol Exp Neurol. 1967 Oct;26(4):521–536. doi: 10.1097/00005072-196710000-00001. [DOI] [PubMed] [Google Scholar]
- Williams V., Grossman R. G. Ultrastructure of cortical synapses after failure of presynaptic activity in ischemia. Anat Rec. 1970 Feb;166(2):131–141. doi: 10.1002/ar.1091660202. [DOI] [PubMed] [Google Scholar]
- Yamamoto C., Bak I. J., Kurokawa M. Ultrastructural changes associated with reversible and irreversible suppression of electrical activity in olfactory cortex slices. Exp Brain Res. 1970 Nov 26;11(4):360–372. doi: 10.1007/BF00237909. [DOI] [PubMed] [Google Scholar]