Abstract
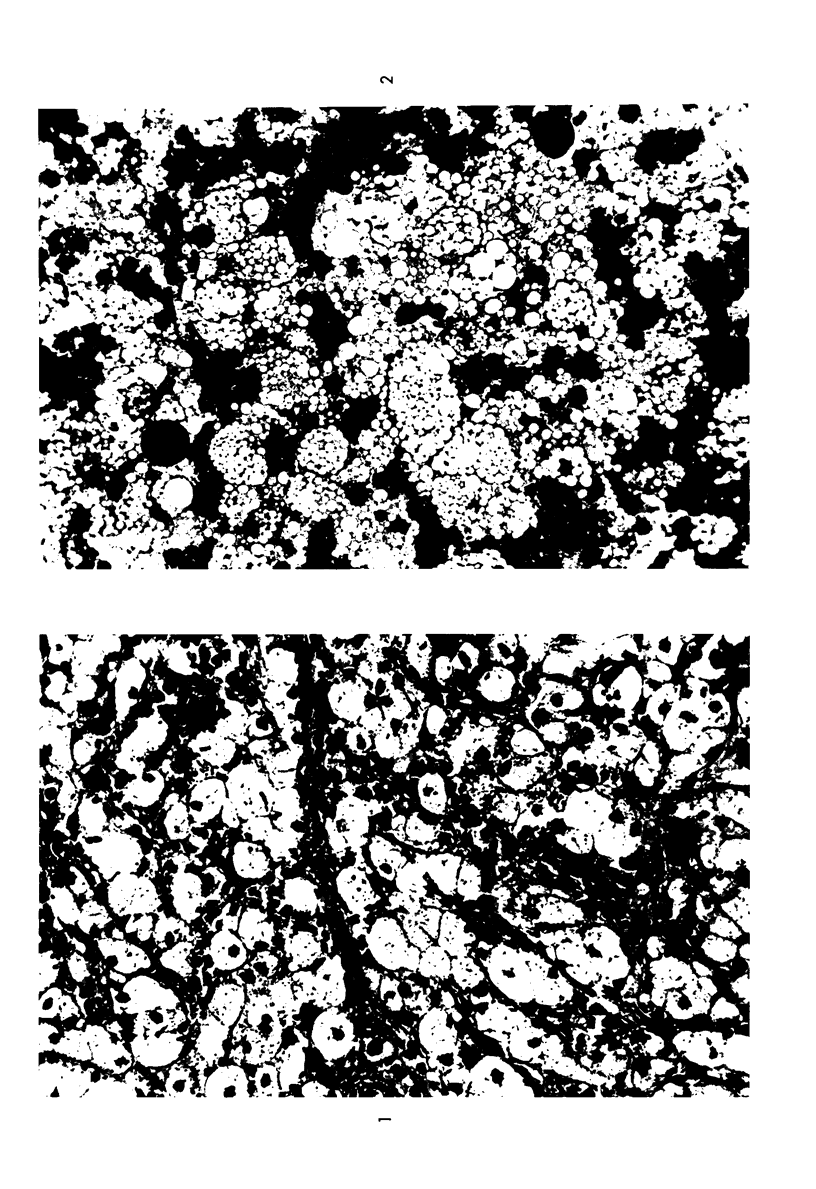
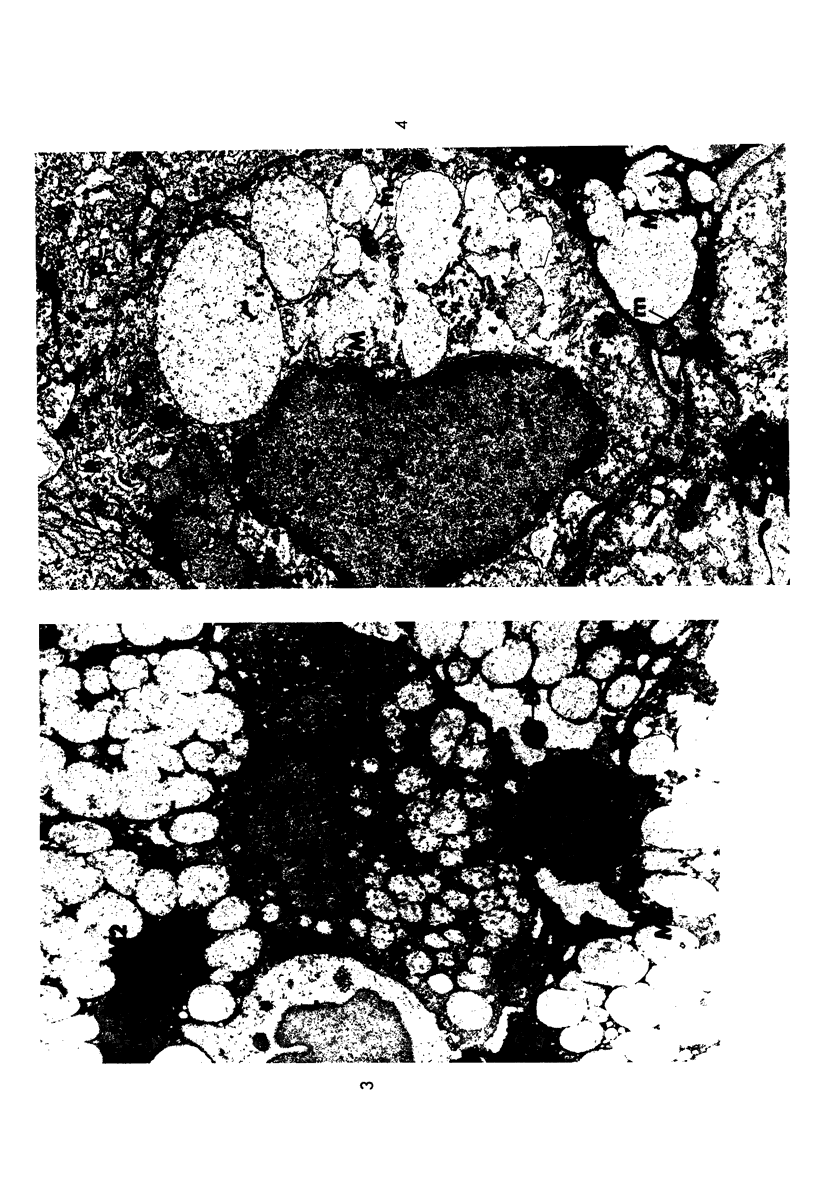
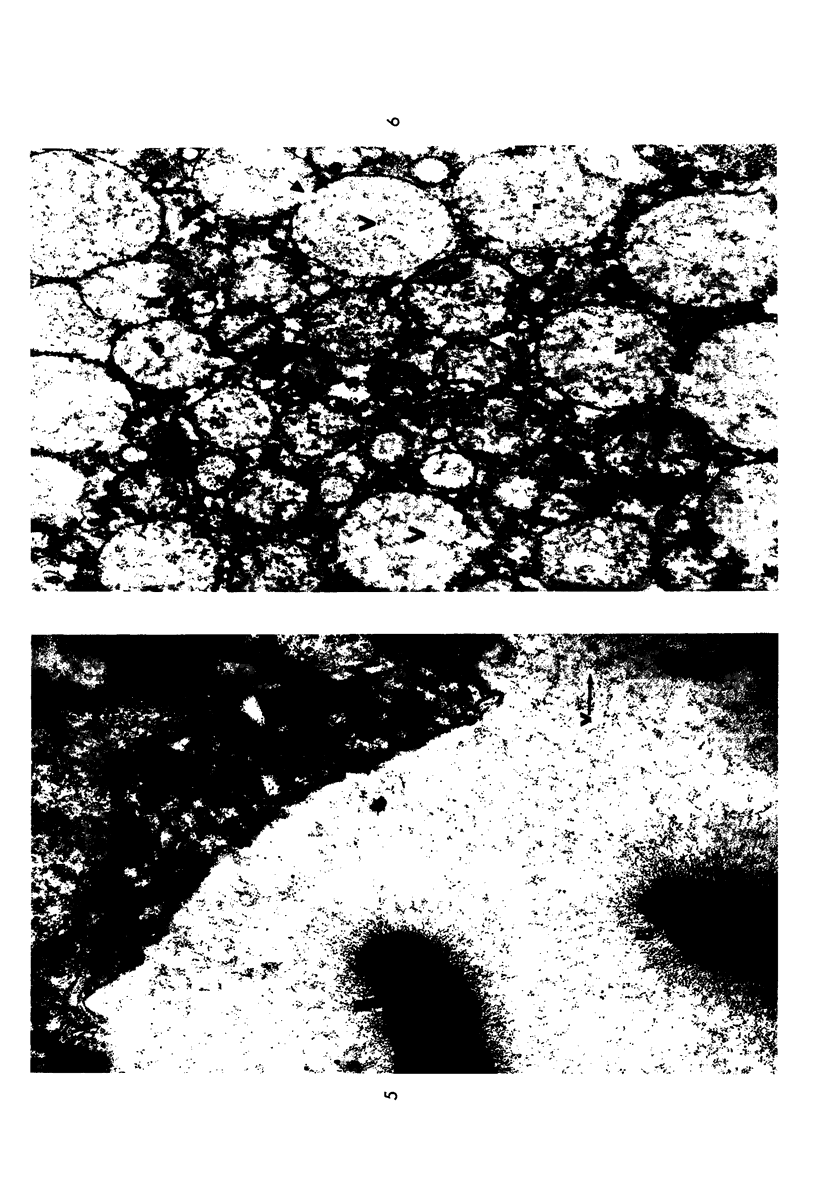
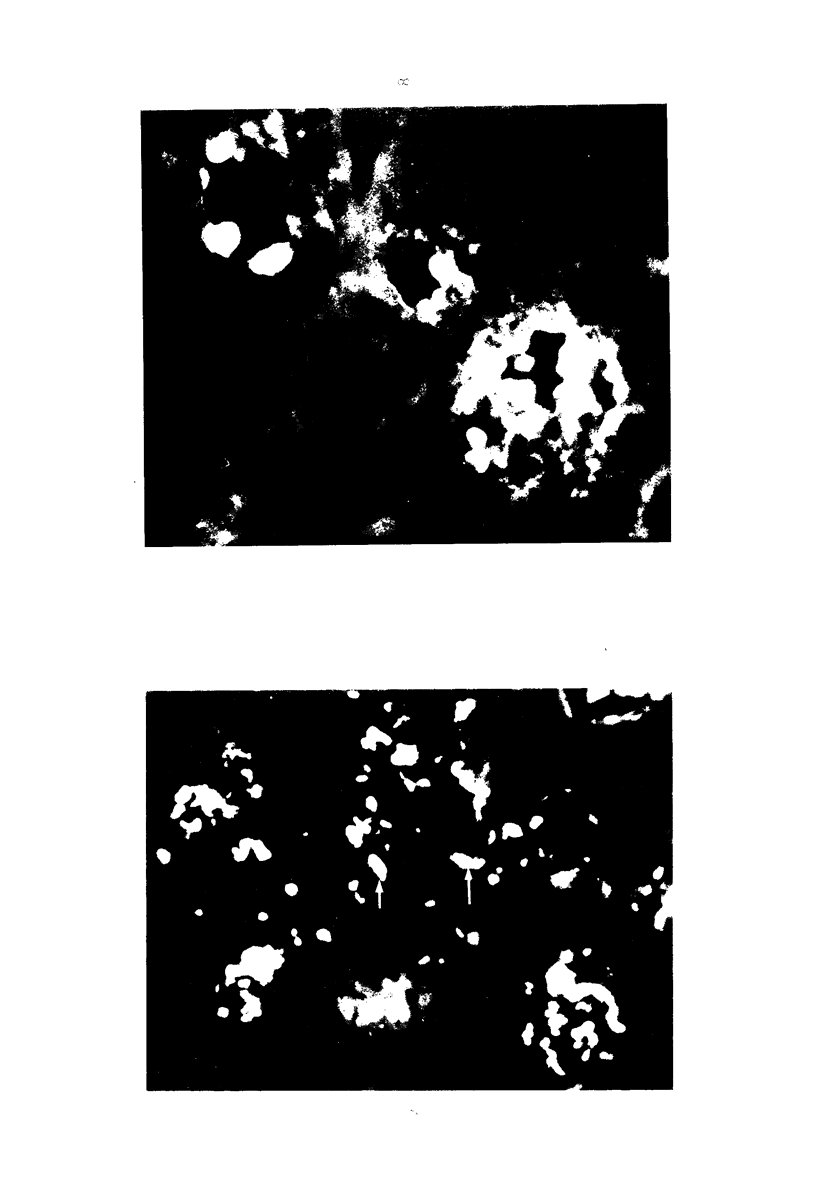
The stages in the development of the Mikulicz cell in human rhinoscleroma were studied in biopsy specimens obtained from 10 patients using light, immunofluorescent and electron microscopy. The Mikulicz cell was identified morphologically as a macrophage, not a plasma cell. Acutely inflamed areas of rhinoscleroma presented abundant bacteria with a slime layer. The microorganism was infrequent and the mucopolysaccharide was scanty in rhinoscleromal tissue, where plasma cells predominated, and in cicatricial fibrous tissue. In the granulomatous stage of rhinoscleroma, the mucopolysaccharide was found within the Mikulicz cells. The vacuoles observed in the Mikulicz cells were considered to be phagosomes containing, principally, bacterial mucopolysaccharide and few bacteria and, to a lesser extent, swollen mitochondria. It was concluded that the slime layer of Klebsiella rhinoscleromatis plays an important role in the pathogenesis of the disease. It is postulated that this material is a nondigestible mucopolysaccharide that resides in the phagosomes of macrophages, increases the osmotic pressure and forms multiple hydropic vacuoles that rupture not only the phagosomes but also the cells, resulting in the liberation of the mucopolysaccharide. This would initiate a cycle that would prolong the disease in the absence of the bacteria.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong B. A., Sword C. P. Electron microscopy of Listeria monocytogenes-infected mouse spleen. J Bacteriol. 1966 Mar;91(3):1346–1355. doi: 10.1128/jb.91.3.1346-1355.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTEN J. J. Scleroma respiratorium in Indonesia, particularly the scleroma endemic in North Celebes. Doc Med Geogr Trop. 1956 Jun;8(2):101–116. [PubMed] [Google Scholar]
- Cohn Z. A., Ehrenreich B. A. The uptake, storage, and intracellular hydrolysis of carbohydrates by macrophages. J Exp Med. 1969 Jan 1;129(1):201–225. doi: 10.1084/jem.129.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G. A., EDWARDS M. R., HAZEN E. L. Electron microscopic study of Histoplasma in mouse spleen. J Bacteriol. 1959 Apr;77(4):429–438. doi: 10.1128/jb.77.4.429-438.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER E. R., DIMLING C. RHINOSCLEROMA. LIGHT AND ELECTRON MICROSCOPIC STUDIES. Arch Pathol. 1964 Nov;78:501–512. [PubMed] [Google Scholar]
- Furnas D. W. Recognition of scleroma (rhinoscleroma). Laryngoscope. 1968 Nov;78(11):1948–1952. doi: 10.1288/00005537-196811000-00009. [DOI] [PubMed] [Google Scholar]
- Godoy G. A. Identificación de Klebsiella rhinoscleromatis en frotis de cultivos puros y tejidos de ratón utilizando técnicas de anticuerpos fluorescentes. Rev Latinoam Microbiol Parasitol (Mex) 1966 Jul-Sep;8(3):119–127. [PubMed] [Google Scholar]
- Gonzalez-Angulo A., Marques-Monter H., Greenberg S. D., Cerbon J. Ultrastructure of nasal scleroma (emphasizing the fine structure of Klebsiella rhinoscleromatis within the lesion). Ann Otol Rhinol Laryngol. 1965 Dec;74(4):1022–1033. doi: 10.1177/000348946507400409. [DOI] [PubMed] [Google Scholar]
- Imaeda T. Electron microscopy. Approach to leprosy research. Int J Lepr. 1965 Jul-Sep;33(3 Suppl):669–688. [PubMed] [Google Scholar]
- KARLSBAD G., KESSEL R. W., DE PETRIS S., MONACO L. ELECTRON MICROSCOPE OBSERVATIONS OF BRUCELLA ABORTUS GROWN WITHIN MONOCYTES IN VITRO. J Gen Microbiol. 1964 Jun;35:383–390. doi: 10.1099/00221287-35-3-383. [DOI] [PubMed] [Google Scholar]
- Miller N. G., Wilson R. B. IN VIVO AND IN VITRO OBSERVATIONS OF LEPTOSPIRA POMONA BY ELECTRON MICROSCOPY. J Bacteriol. 1962 Sep;84(3):569–576. doi: 10.1128/jb.84.3.569-576.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyka M. M., Gubina K. M. Problems of the epidemiology of scleroma. 2. Some aspects of the problem of endemic focus formation. J Hyg Epidemiol Microbiol Immunol. 1972;16(1):8–20. [PubMed] [Google Scholar]
- Rojas-Espinosa O., Estrada-Parra S. Inmunochemistry of the Capsular polysaccharide of Klebsiella rhinoscleromatis. Rev Latinoam Microbiol Parasitol (Mex) 1968 Jan-Mar;10(1):7–10. [PubMed] [Google Scholar]
- SHAW H. J., MARTIN H. Rhinoscleroma--a clinical perspective. J Laryngol Otol. 1961 Dec;75:1011–1039. doi: 10.1017/s0022215100058837. [DOI] [PubMed] [Google Scholar]
- Shokeir A. A., Osman M. Rhinoscleroma, an electron-microscopy study. J Hyg Epidemiol Microbiol Immunol. 1972;16(1):1–7. [PubMed] [Google Scholar]
- WELSH R. A., CORREA P., HERRAN R. Light and electron microscopic observations of scleroma. Exp Mol Pathol. 1963 Feb;2:93–101. doi: 10.1016/0014-4800(63)90011-x. [DOI] [PubMed] [Google Scholar]
- WHEAT R. W., DORSCH C., GODOY G. OCCURRENCE OF PYRUVIC ACID IN THE CAPSULAR POLYSACCHARIDE OF KLEBSIELLA RHINOSCLEROMATIS. J Bacteriol. 1965 Feb;89:539–539. doi: 10.1128/jb.89.2.539-539.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyke S., Domagala W., Olszewski W. Electron microscopic studies of scleroma granulation tissue. Acta Med Pol. 1969;10(2):231–242. [PubMed] [Google Scholar]