Abstract
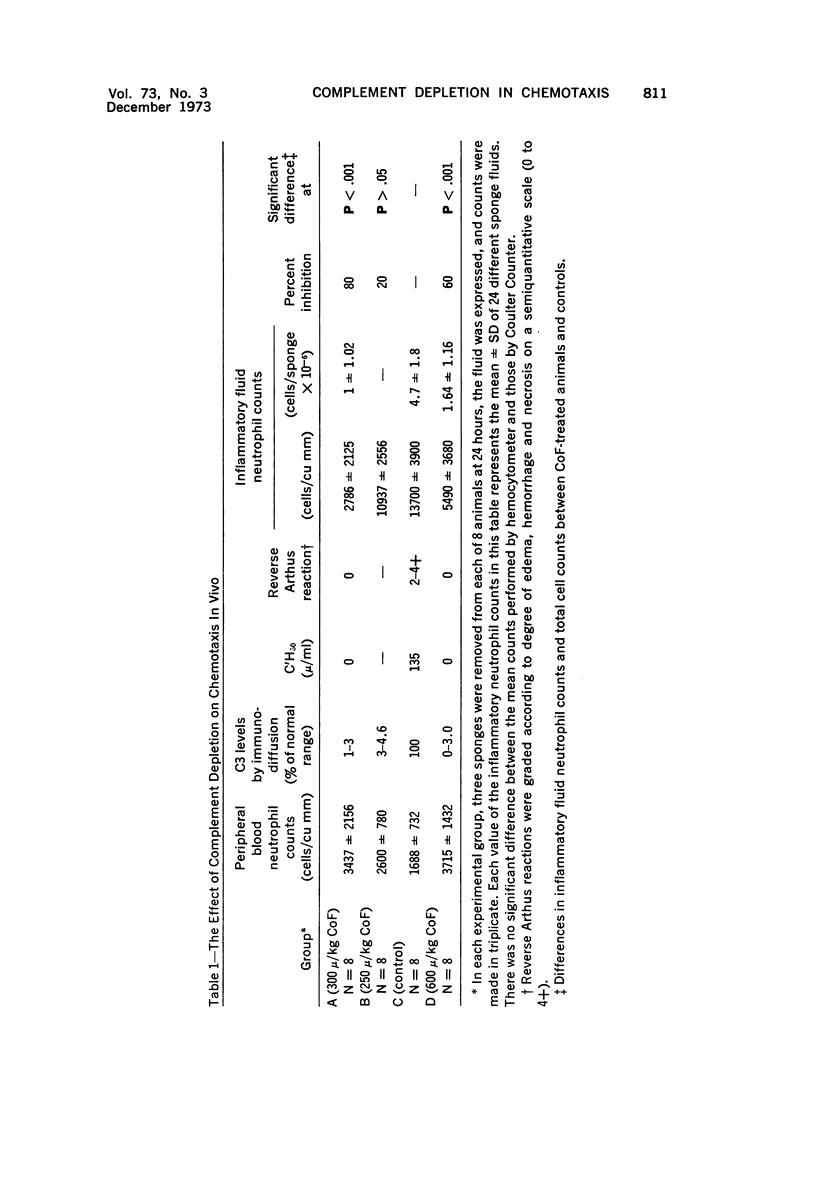
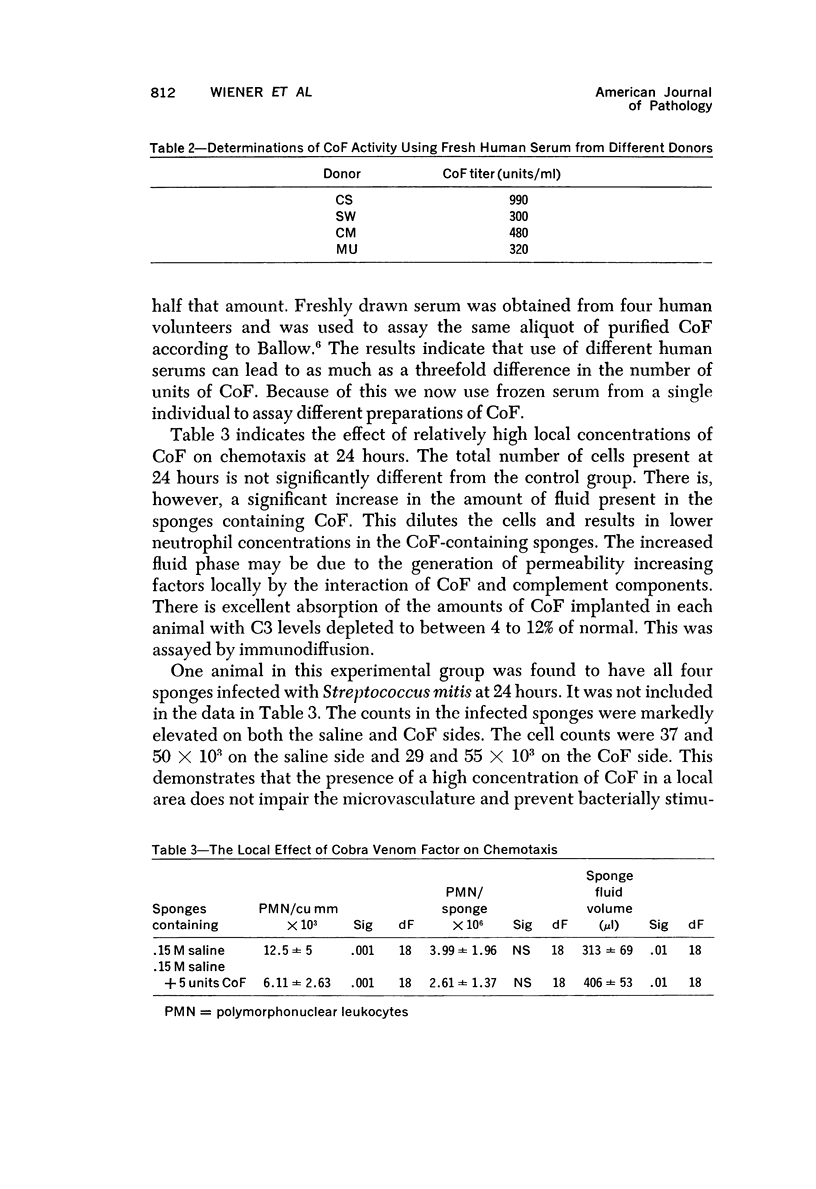
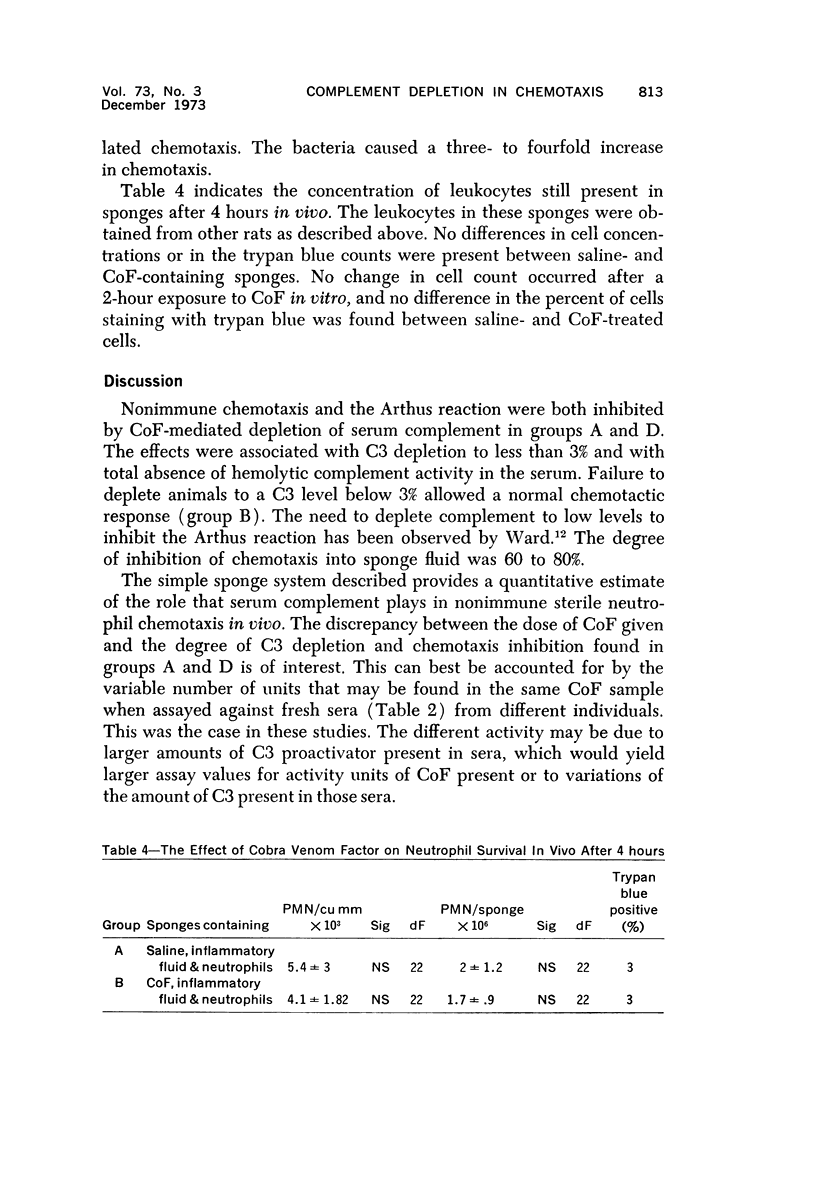
Wistar rats were depleted of complement components using purified cobra venom factor (CoF) administered over a 30-hour period by intraperitoneal injection. Complement depletion was confirmed by immunodiffusion assay, hemolytic assay and inhibition of the reverse Arthus reaction. Rats depleted to below 3% of their normal serum complement level showed marked inhibition of chemotaxis into inflammatory fluid produced in polyvinyl sponges 24 hours after implantation into the dorsal subcutaneous region. Subsequent studies were done to test the effect of high local concentrations of CoF on the microcirculation, the neutrophil and the connective tissue. Local exposure of these tissues and cells to CoF in vivo had no inhibiting effect on chemotaxis. Cobra venom factor did not affect neutrophil survival in vitro or in vivo. The most likely hypothesis is that CoF works by depleting complement and that in vivo chemotaxis of the nonbacterial, nonimmune type is complement dependent to the extent of 60 to 80%. The system described allows for quantitative determination of chemotaxis in vivo.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOUCEK R. J., NOBLE N. L. Connective tissue; a technique for its isolation and study. AMA Arch Pathol. 1955 May;59(5):553–558. [PubMed] [Google Scholar]
- Ballow M., Cochrane C. G. Two anticomplementary factors in cobra venom: hemolysis of guinea pig erythrocytes by one of them. J Immunol. 1969 Nov;103(5):944–952. [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Hill J. H., Ward P. A. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med. 1971 Apr 1;133(4):885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaley G., Weiner R. Effect of prostaglandin E 1 on leukocyte migration. Nat New Biol. 1971 Nov 24;234(47):114–115. doi: 10.1038/newbio234114a0. [DOI] [PubMed] [Google Scholar]
- MARDINEY M. R., Jr, MUELLER-EBERHARD H. J. MOUSE BETA-1C-GLOBULIN: PRODUCTION OF ANTISERUM AND CHARACTERIZATION IN THE COMPLEMENT REACTION. J Immunol. 1965 Jun;94:877–882. [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Ward P. A. Neutrophil chemotactic factors and related clinical disorders. Arthritis Rheum. 1970 Mar-Apr;13(2):181–186. doi: 10.1002/art.1780130210. [DOI] [PubMed] [Google Scholar]


