Abstract
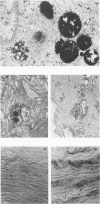
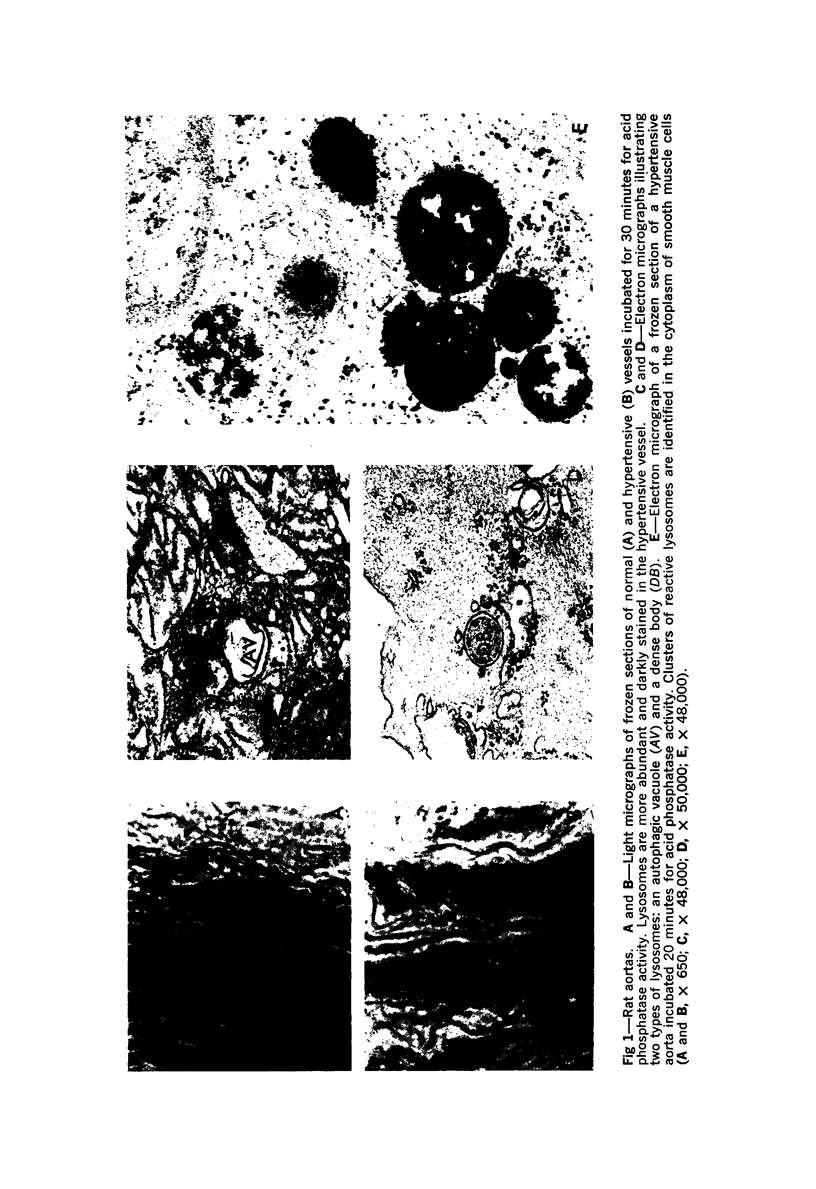
Hypertension induces hypertrophy and increased turnover of aortic smooth muscle cells along with an accumulation of connective tissue in the aortic wall. We identified the lysosomes in normal and hypertensive aortic muscle cells by light and electron microscopy, utilizing cytochemical staining for acid phosphatase activity. Lysosomes were found to be more numerous in hypertensive vessels. Biochemical assays of two specific lysosomal enzymes revealed a doubling of acid phosphatase and a more than threefold increase in β-N-acetyl-glucosaminidase activities in hypertensive aortas.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CERIOTTI G. A microchemical determination of desoxyribonucleic acid. J Biol Chem. 1952 Sep;198(1):297–303. [PubMed] [Google Scholar]
- Curreri P. W., Kothari H. V., Bonner M. J., Miller B. F. Increased activity of lysosomal enzymes in experimental atherosclerosis, and the effect of cortisone. Proc Soc Exp Biol Med. 1969 Apr;130(4):1253–1256. doi: 10.3181/00379727-130-33766. [DOI] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Fernandez D., Crane W. A. New cell formation in rats with accelerated hypertension due to partial aortic constriction. J Pathol. 1970 Apr;100(4):307–316. doi: 10.1002/path.1711000410. [DOI] [PubMed] [Google Scholar]
- Gardner D. L., Matthews M. A. Ultrastructure of the wall of small arteries in early experimental rat hypertension. J Pathol. 1969 Jan;97(1):51–62. doi: 10.1002/path.1710970108. [DOI] [PubMed] [Google Scholar]
- Hollander W., Kramsch D. M., Farmelant M., Madoff I. M. Arterial wall metabolism in experimental hypertension of coarctation of the aorta of short duration. J Clin Invest. 1968 May;47(5):1221–1229. doi: 10.1172/JCI105811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R. W., Matthew W. T., Dubowik D. A. Factors influencing the determination of DNA with indole. Anal Biochem. 1970 Nov;38(1):190–201. doi: 10.1016/0003-2697(70)90169-7. [DOI] [PubMed] [Google Scholar]
- Hüttner I., More R. H., Rona G. Fine structural evidence of specific mechanism for increased endothelial permeability in experimental hypertension. Am J Pathol. 1970 Dec;61(3):395–412. [PMC free article] [PubMed] [Google Scholar]
- Kojimahara M., Ooneda G. Electron microscopic study on the middle cerebral artery lesions in hypertensive rats. Acta Pathol Jpn. 1970 Nov;20(4):399–408. doi: 10.1111/j.1440-1827.1970.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Miller B. F., Kothari H. V. Increased activity of lysosomal enzymes in human atherosclerotic aortas. Exp Mol Pathol. 1969 Jun;10(3):288–294. doi: 10.1016/0014-4800(69)90058-6. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt D. Hyaluronidase-, beta-Glucuronidase- und beta-Acetylglucosaminidase-Aktivität in normalen und arteriosklerotisch veränderten menschlichen Aorten. Klin Wochenschr. 1967 Jan 15;45(2):92–95. doi: 10.1007/BF01747970. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. F., Jones R., Daoud A. S., Zumbo O., Coulston F., Thomas W. A. Experimental atherosclerosis in rhesus monkeys. II. Cellular elements of proliferative lesions and possible role of cytoplasmic degeneration in pathogenesis as studied by electron microscopy. Exp Mol Pathol. 1967 Aug;7(1):34–57. doi: 10.1016/0014-4800(67)90037-8. [DOI] [PubMed] [Google Scholar]
- Tobian L., Olson R., Chesley G. Water content of arteriolar wall in renovascular hypertension. Am J Physiol. 1969 Jan;216(1):22–24. doi: 10.1152/ajplegacy.1969.216.1.22. [DOI] [PubMed] [Google Scholar]
- Wiener J., Lattes R. G., Meltzer B. G., Spiro D. The cellular pathology of experimental hypertension. IV. Evidence for increased vascular permeability. Am J Pathol. 1969 Feb;54(2):187–207. [PMC free article] [PubMed] [Google Scholar]
- Wolinsky H., Daly M. M. A method for the isolation of intima-media samples from arteries. Proc Soc Exp Biol Med. 1970 Nov;135(2):364–368. doi: 10.3181/00379727-135-35052. [DOI] [PubMed] [Google Scholar]
- Wolinsky H. Effects of hypertension and its reversal on the thoracic aorta of male and female rats. Morphological and chemical studies. Circ Res. 1971 Jun;28(6):622–637. doi: 10.1161/01.res.28.6.622. [DOI] [PubMed] [Google Scholar]