Abstract
G protein-coupled receptors mediate biological signals by stimulating nucleotide exchange in heterotrimeric G proteins (Gαβγ). Receptor dimers have been proposed as the functional unit responsible for catalytic interaction with Gαβγ. To investigate whether a G protein-coupled receptor monomer can activate Gαβγ, we used the retinal photoreceptor rhodopsin and its cognate G protein transducin (Gt) to determine the stoichiometry of rhodopsin/Gt binding and the rate of catalyzed nucleotide exchange in Gt. Purified rhodopsin was prepared in dodecyl maltoside detergent solution. Rhodopsin was monomeric as concluded from fluorescence resonance energy transfer, copurification studies with fluorescent labeled and unlabeled rhodopsin, size exclusion chromatography, and multiangle laser light scattering. A 1:1 complex between light-activated rhodopsin and Gt was found in the elution profiles, and one molecule of GDP was released upon complex formation. Analysis of the speed of catalytic rhodopsin/Gt interaction yielded a maximum of ≈50 Gt molecules per second and molecule of activated rhodopsin. The bimolecular rate constant is close to the diffusion limit in the diluted system. The results show that the interaction of Gt with an activated rhodopsin monomer is sufficient for fully functional Gt activation. Although the activation rate in solution is at the physically possible limit, the rate in the native membrane is still 10-fold higher. This is likely attributable to the precise orientation of the G protein to the membrane surface, which enables a fast docking process preceding the actual activation step. Whether docking in membranes involves the formation of rhodopsin dimers or oligomers remains to be elucidated.
Keywords: enzyme catalysis, nucleotide exchange, light scattering
G protein-coupled receptors (GPCRs) constitute the largest group of transmembrane receptors. These seven-transmembrane helix proteins are involved in detecting a wide variety of chemical and physical stimuli, ranging from ligand binding to light perception (1). In their active state, GPCRs catalyze nucleotide exchange in heterotrimeric G proteins (Gαβγ) and thereby route extracellular signals to distinct intracellular signaling pathways (2). Rhodopsin is the prototype and eponym of the largest class of GPCRs, the rhodopsin-like GPCRs (class A). Together with its cognate G protein transducin (Gt), it is part of the visual signaling system found on the disk membranes in the outer segment of vertebrate rod photoreceptor cells. Rhodopsin is activated by photon absorption, causing cis-trans isomerization of the chromophore 11-cis-retinal, which is bound covalently to the apoprotein opsin. Optimal photon absorption of photoreceptor cells is ensured by the high density of rhodopsin in the disk membranes [>25,000 receptors per μm2 (3)].
In disk membranes on mica support, rows of rhodopsin dimers were observed by atomic force microscopy (4). Different types of dimers also were found in crystal structures of rhodopsin (see ref. 5 and references therein). Such direct structural analysis of a GPCR currently is available only for rhodopsin. However, other GPCRs also were found to associate as dimers and higher-order oligomers in living cells (see, e.g., refs. 6–8), although monomers prevail at low expression levels (9, 10). Molecular modeling (11–13), FRET measurements (14–16), and biochemical approaches (5, 17, 18) are consistent with a model in which dimers or higher-order oligomers of rhodopsin, or class A GPCRs in general, constitute the functional unit for G protein activation (19–21). Although this paradigm has been questioned (10, 22, 23), a supramolecular organization of GPCRs indeed may enhance the performance and fidelity with which signals are transferred to the G protein in signal transduction modules (11, 24). Using a simplified system, namely monomeric rhodopsin in detergent solution, we have tested whether such organization of rhodopsin is necessary for efficient catalytic activation of Gt, i.e., nucleotide exchange in the α-subunit of Gt by interaction with light-activated rhodopsin (R*).
Results
Preparation of Monomeric Rhodopsin in Detergent Solution.
Rhodopsin used in this study was either prepared from bovine disk membranes or expressed as its apoprotein opsin in COS-1 cells and reconstituted with 11-cis-retinal. For solubilization and purification of rhodopsin, we used n-dodecyl-β-d-maltopyranoside (DDM) detergent and an immunoaffinity procedure, respectively. We found that the obtained rhodopsin was monomeric, as shown by several lines of evidence:
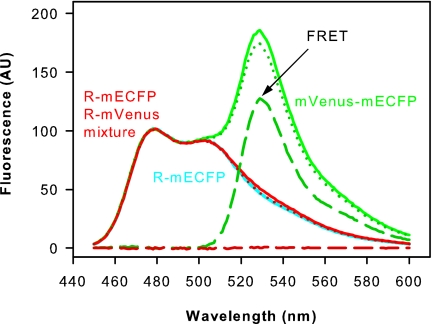
No FRET was observed when opsin apoproteins fused to monomeric variants of enhanced cyan fluorescent protein (mECFP) or yellow fluorescent protein (mVenus), respectively, were coexpressed in COS-1 cells, reconstituted with 11-cis-retinal, and copurified in DDM solution. In contrast, a positive FRET control consisting of a purified mVenus–mECFP fusion showed FRET (Fig. 1). Also, when expressed in COS-1 cells, fluorescent opsin fusion proteins in the cell membrane showed FRET (15), indicating a quaternary structure in these membranes.
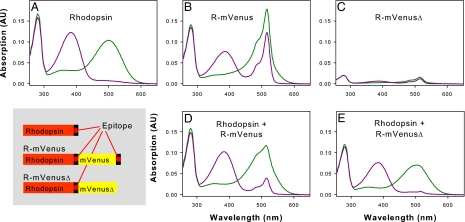
To verify that our purification protocol yielded monomeric rhodopsin, we performed coexpression and copurification with rhodopsin and the rhodopsin–mVenus fusion protein (Fig. 2). Using unmodified rhodopsin as a purification handle, it was not possible to copurify rhodopsin–mVenus fusion protein lacking a C-terminal affinity tag, which argues again for rhodopsin monomers in solution.
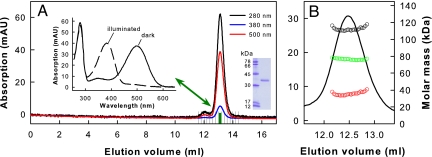
The immunoaffinity purification protocol then was used to purify native rhodopsin from bovine rod cells. Size exclusion chromatography (SEC) of the purified rhodopsin revealed high homogeneity and purity of the preparation as indicated by the absorption spectrum (Fig. 3A).
Further analysis by SEC in combination with multiangle laser light scattering (MALLS) detection showed that only monomers were present in the main elution peak of dark state rhodopsin (Fig. 3B). MALLS evaluation of each elution slice (Fig. 3B) revealed for the central part of the elution peak that protein monomers (experimental mass ≈35 kDa; theoretical mass ≈39 kDa; Fig. 3B, red circles) are enclosed by a detergent micelle on the scale of ≈74 kDa (Fig. 3B, green circles), yielding a rhodopsin–detergent complex of ≈110 kDa (Fig. 3B, black circles). The monomeric nature of rhodopsin remained unaffected after light-activating it directly before the SEC run.
Fig. 1.
FRET experiments. Monomeric cyan or yellow variants of GFP fused to the opsin C terminus (R–mECFP and R–mVenus, respectively) were expressed separately and together in COS-1 cells, reconstituted with 11-cis-retinal, and purified. Normalized emission spectra (excitation at 420 nm) are shown for purified R–mECFP (cyan line), copurified R–mECFP/R–mVenus (red line), and a fusion protein consisting of mECFP and mVenus as positive FRET control (green line). The spectra of proteins containing an mVenus moiety were corrected for direct excitation of mVenus by the 420 nm excitation beam as described in Materials and Methods. FRET is reflected in the difference between the corrected spectra (dotted lines) and the spectrum of R–mECFP (cyan line). These difference spectra (broken lines) show that FRET only occurs in the mVenus–mECFP positive FRET control but not in the R–mECFP/R–mVenus sample. FRET also was lacking in copurified R–mECFP/R–mVenus after illumination of the sample for 15 s with orange light (data not shown).
Fig. 2.
UV/visible absorption spectra of rhodopsin and different rhodopsin–mVenus fusion proteins after expression in COS-1 cells, reconstitution with 11-cis-retinal and purification. The proteins contained one or two epitopes (internal and/or C-terminal) recognized by rho-1D4 antibody used for immunoaffinity purification. Spectra measured in the dark (green line) and after illumination for 15 s with orange light (purple line) are shown. (A) Rhodopsin. (B) R–mVenus. (C) R–mVenusΔ. (D) Copurified rhodopsin and R–mVenus (1:1 molar ratio of plasmids used for transfection). (E) Copurified rhodopsin and R–mVenusΔ (1:2 molar ratio of plasmids used for transfection). The small contribution of mVenus to the absorption at ≈516 nm is attributable to the weak affinity of the rho-1D4 antibody for the internal epitope (see C). The results show that fluorescent-protein-tagged rhodopsin lacking the C-terminal epitope tag does not copurify with coexpressed wild-type rhodopsin. Analogously, coexpression of R–mVenus and wild-type rhodopsin lacking the 1D4 epitope yielded spectra very similar to R–mVenus alone (data not shown).
Fig. 3.
SEC of rhodopsin purified from bovine disk membranes. (A) Typical chromatogram of rhodopsin purified in DDM detergent. The peak fraction highlighted in green was used for subsequent Gt activation experiments. Inset shows UV/visible absorption spectra of the highlighted fraction measured in the dark and after illumination for 15 s with orange light. This rhodopsin fraction typically had an A280/A500 ratio below 1.6, indicating a purity close to 100%. (Inset) SDS gel of purified rhodopsin (right lane). (B) Online molar mass determination of purified dark-state rhodopsin in 0.03% (wt/vol) DDM by SEC in combination with MALLS detection. The calculated molar masses are shown as a function of elution volume for the rhodopsin–detergent complex (black circles). The calculated molar masses of protein and detergent in the rhodopsin–detergent complex are shown as red and green circles, respectively. The values obtained (35 kDa and 74 kDa) are close to the molar masses of rhodopsin (39 kDa; determined from the primary structure) and DDM micelles [74 kDa (49)].
Stoichiometry of Interaction Between Monomeric Rhodopsin and Transducin.
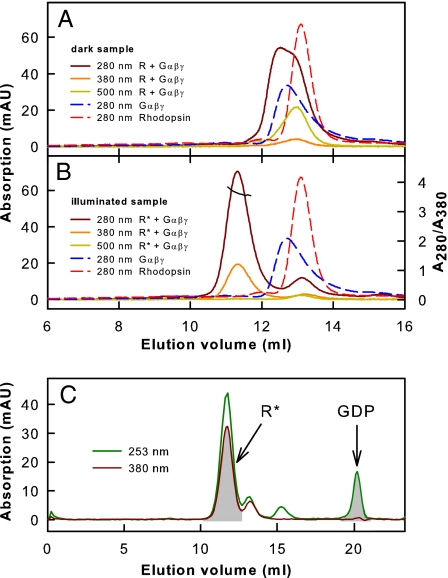
We analyzed the interaction of monomeric rhodopsin with Gt by SEC (Fig. 4). SEC first was performed with inactive dark state rhodopsin mixed with inactive GDP-bound Gt (Fig. 4A). To study the interaction of R* with Gt, the mixture of rhodopsin and Gt was illuminated with orange light immediately before SEC to produce R*. Interaction between R* and Gt was observed as a shift of the elution peak to a lower elution volume attributable to R*·Gt complex formation (Fig. 4B). The different absorption maxima of proteins (280 nm) and retinal chromophore (380 nm; as part of R*) allowed us to determine a stoichiometry of 1:1 for R* and Gt in the main peak containing the protein complex (see Fig. 4 legend and Materials and Methods for details). The equimolar R*:Gt stoichiometry was confirmed further by analysis of GDP release upon R*·Gt complex formation (Fig. 4C). The amount of R*·Gt complex formed is reflected in the area of the elution peak detected at 380 nm (absorption maximum of R*; Fig. 4C). Analogously, the area of the GDP elution peak detected at 253 nm (absorption maximum of GDP) reflects the amount of GDP released upon R*·Gt complex formation. Normalization of the peak areas by the different extinction coefficients of R* and GDP yielded approximately the same values, i.e., equimolar amounts of R* and GDP (see figure legend and Materials and Methods for details). Accordingly, one GDP molecule was released from Gt·GDP upon interaction with R*, again consistent with a 1:1 R*·Gt complex.
Fig. 4.
Transducin binding to monomeric rhodopsin. (A) Superposition of SEC elution profiles: Gt holoprotein (Gαβγ) or rhodopsin (R; inactive ground state) alone (dashed lines) or as mixture (solid lines). (B) SEC elution profiles as in A obtained with R*. Detection occurred at the absorption maxima of protein (280 nm), R* (380 nm), and rhodopsin (500 nm). R*·Gt complex formation is indicated by a shift of the main elution peak. For this peak, a value of 3.6–3.8 was determined for the absorption ratio A280/A380 (black line), which is consistent with a 1:1 stoichiometry between R* and Gt in the R*·Gt complex (theoretical values are 3.56 for R*:Gt = 1:1 and 2.52 for R*:Gt = 2:1; see Materials and Methods). (C) SEC elution profile of R*/Gt mixture detected at 380 nm (absorption maximum of R*) and 253 nm (absorption maximum of GDP). The ratio between peak areas corresponding to R* and GDP, respectively, ranges from 3.0 to 3.2 for different runs (theoretical values are 3.07 for R*:Gt = 1:1 and 6.13 for R*:Gt = 2:1; see Materials and Methods).
Analysis of Gt Activation by Monomeric Rhodopsin.
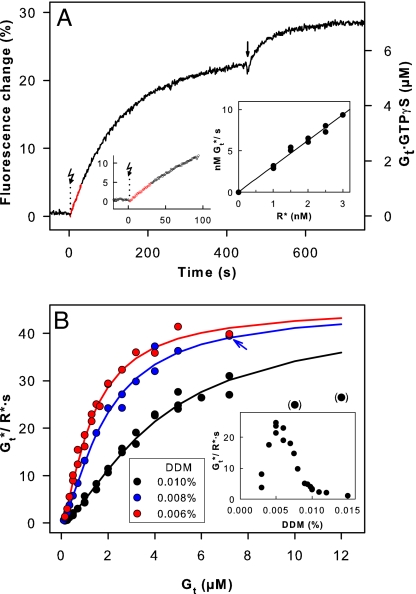
For Gt activation measurements, we used the central fraction of the SEC elution peak, shown above to contain pure monomeric rhodopsin (Fig. 3A). To quantify the catalytic capacity of monomeric rhodopsin, we determined the initial rate of Gt activation by monitoring the intrinsic tryptophan fluorescence change of the G protein α-subunit, linked to GTPγS uptake (Fig. 5A). We first measured the activation rate [expressed as activated Gt molecules (Gt*) per second] as a function of rhodopsin concentration (R* = 0–3.0 nM; Fig. 5A Inset). The found linear dependence allowed the measured activation rates to be normalized to R*. To obtain the maximum velocity (Vmax) of the reaction, we titrated the fluorescence changes with G protein up to a concentration of 12 μM Gt. The highest activation rate measured was ≈40 Gt*/R*·s (Fig. 5B). This is a lower limit because at higher Gt concentrations the rate decreased. Titration curves significantly deviate from the simple Michaelis–Menten type of hyperbolic curve, because of subunit dissociation at low Gt concentration and inhibition of R*/Gt interaction by the detergent (Fig. 5B). Gt interacts with detergent micelles because of its hydrophobic modifications, i.e., myristoylation of the α-subunit and farnesylation of the γ-subunit (25). Consistently, highest activation rates were obtained close to the critical micelle concentration of DDM [≈0.005% (wt/vol); Fig. 5B Inset]. In agreement with this finding, recombinant nonmyristoylated Gαi1/Gtβγ showed little detergent sensitivity (data not shown). At 0.006% (wt/vol) DDM, hyperbolic saturation was approached. Under these conditions, a Michaelis constant (Km) of 0.55 μM was obtained when accounting for the detergent effect and Gt subunit dissociation [see Materials and Methods and supporting information (SI) Methods for details]. A maximal velocity (Vmax) of 46 Gt*/R*·s was obtained by global fit to all data points (Fig. 5B). The enzymatic efficiency can be expressed as the ratio of the catalytic rate (kcat) and Km. The value obtained from our data is kcat/Km = Vmax/(R*·Km) = 8.4·107 M−1·s−1, which is close to the diffusion limit expected for the encounter of two proteins in solution (26) and shows that the catalytic interaction between monomeric rhodopsin and its G protein is very efficient.
Fig. 5.
G protein activation by monomeric rhodopsin. (A) Activation of Gt measured as tryptophan fluorescence increase attributable to GTPγS uptake [shown for 7.2 μM Gt, 1 nM R*, 200 μM GTPγS, and 0.008% (wt/vol) DDM at 20°C and pH 7.1]. Reactions were triggered by light activation (flash symbol) of rhodopsin. The arrow marks the point when additional R* was added to activate the whole Gt pool. (Left Inset) Same signal on expanded scale. Data points plotted in red were used for linear regression to determine reaction rate from initial slope of the fluorescence trace. See SI Fig. 6 for control experiments. (Right Inset) Reaction rates [nM activated Gt (Gt*) per second] as function of R* concentration [1 μM Gt, 0–3.0 nM R*, 0.01% (wt/vol) DDM]. (B) Gt activation rates plotted as a function of total Gt concentration [DDM concentration (wt/vol) as indicated]. Blue arrow indicates the data point (blue dot behind red dot) from the trace in A. Solid lines are best least-square fits of the data to a Michaelis–Menten type hyperbolic function, taking Gt subunit dissociation and Gt binding to detergent micelles into account. The numerical global fit yielded for all curves Vmax = 46 Gt*/R*·s, K1 = 0.46 μM (Gt subunit dissociation), and K2 = 1.76 μM (Gt micelle binding). Km values are 0.55 μM (0.006% DDM), 0.8 μM (0.008% DDM), and 2.3 μM (0.01% DDM). For recombinant rhodopsin and Gt, a similar rate profile was obtained [0.01% (wt/vol) DDM; data not shown]. (Inset) Gt activation rates (1 μM Gt) as a function of DDM detergent concentration.
Discussion
Our results show that in DDM detergent solution rhodopsin interacts with its cognate G protein Gt in a 1:1 stoichiometry and that the rhodopsin monomer also is competent to activate Gt with a rate close to the physical limit in solution. The maximum rate in the native membrane still is about one order of magnitude higher than in solution (27, 28). This finding may be explained by the reduction in translational degrees of freedom in a two-dimensional membrane, which facilitates successful coupling. Fast and immediate docking is enabled by the precise orientation of the G protein to the membrane surface. The membrane anchor of the G protein made up of the myristoyl/farnesyl modifications at the α- and γ-subunits, respectively (25, 29, 30), now can play a decisive role in the initial encounter between the proteins. Evidence for a sequential interaction between different binding sites on Gt and R*, which includes such a docking process, recently was presented (30, 31). Computational modeling suggested a single rhodopsin molecule to be sufficient for docking of Gt (32, 33). It remains to be elucidated whether a pairing of rhodopsin in disk membranes further improves the overall speed of catalytic Gt activation.
Materials and Methods
Materials.
All materials and purified anti-rhodopsin monoclonal antibody rho-1D4 were as described in ref. 34. Buffer A was 20 mM 1,3-bis[Tris(hydroxymethyl)methylamino]propane (pH 7.1), 130 mM NaCl, and 1 mM MgCl2.
Construction of R–mVenus, R–mECFP, and mVenus–mECFP Fusion Plasmids.
The mammalian cell expression vector pMT4 containing a synthetic opsin gene was modified by standard cloning and PCR procedures such that the opsin gene was followed by bases GGATCAACCGGT (the AgeI site is underlined), then followed by the gene corresponding to the monomeric fluorescent proteins mECFP or mVenus and codons corresponding to the amino acid sequence GTETSQVAPA. Rhodopsin's C-terminal sequence ETSQVAPA is recognized by the rho-1D4 antibody used for immunoaffinity purification of the fusion proteins (35). The fluorescent proteins used were variants of GFP. The cyan variant mECFP is Aequorea victoria GFP with mammalian codons and the following additional mutations: K26R, F64L, S65T, Y66W, N146I, M153T, V163A, N164H, H231L, a Val-1a insertion (see Clontech Laboratories, Mountain View, CA, and ref. 36), and A206K. The A206K mutation introduced with the QuikChange kit (Stratagene, La Jolla, CA) causes parental ECFP (weakly dimeric) to monomerize (37). The yellow variant mVenus is Aequorea victoria GFP with mammalian codons and the following additional mutations: F46L, F64L, S65G, V68L, S72A, M153T, V163A, S175G, T203Y, H231L, a Val-1a insertion (38), and A206K [to avoid dimerization (37)]. The control construct mVenus–mECFP was cloned by replacing the EcoRI–SalI fragment of R–mECFP with the mVenus gene. The gene product corresponds to a fusion protein of mVenus and mECFP separated by a 20-aa linker sequence. In the plasmid R–mVenusΔ, bases corresponding to the C-terminal amino acid sequence GTETSQVAPA were deleted. All constructs were verified by DNA sequencing.
Expression and Purification of Rhodopsins.
Rhodopsin, R–mVenus, R–mECFP, and mVenus–mECFP fusion proteins were expressed in COS-1 cells by using a diethylamino-ethyl (DEAE)-dextran transfection procedure. The cells were harvested 72 h after transfection, and 11-cis-retinal was added to reconstitute rhodopsin (34, 39). The expressed proteins were purified by using a rho-1D4–Sepharose immunoaffinity matrix as described (34, 39). All procedures were carried out under dim red light. Briefly, cells were harvested in PBS buffer (137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, and 1.5 mM KH2PO4, pH 7.4) and reconstituted with 11-cis-retinal (30 μM final concentration) for 12 h at 6°C. Cells were solubilized in 1% (wt/vol) DDM for 2–4 h at 6°C and then incubated with rho-1D4–Sepharose matrix overnight. For protein purification, the matrix was washed repetitively in a batch procedure using centrifugation to separate matrix and solution. Wash steps were performed twice with 0.03% (wt/vol) DDM in PBS and then once or twice with 0.03% (wt/vol) DDM in 10 mM 1,3-bis[Tris(hydroxymethyl)methylamino]propane (BTP), pH 6.0. Immunoaffinity-purified protein was eluted from the matrix by adding 150 μM peptide corresponding to the C-terminal 18 aa of rhodopsin (3–4 h, 6°C). The eluted protein was separated from the matrix by centrifugation.
Preparation of Transducin.
Gt was prepared from bovine rod outer segments and in most cases was separated into Gα and Gβγ subunits by chromatography as described in ref. 27. Gt or its separated subunits (8–15 ml) were incubated with 0.5-ml Con A Sepharose 4B beads (GE Healthcare, Barrington, IL) at 6°C for 1 h to remove traces of rhodopsin. Gt or separated Gt subunits then were dialyzed against buffer A containing 2 mM DTT. Dialyzed Gt or Gt subunits were concentrated by ultrafiltration using Centricon YM-10 concentrators (Millipore, Billerica, MA) and stored on ice until use. Protein concentration was determined with Bradford reagent. The final Gt concentration ranged between 30 and 40 μM. To determine the amount of activatable Gt, Gt was titrated with exact amounts of GTPγS (250 nM) in the presence of 500 nM R* under constant stirring in a final volume of 700 μl (40). GTPγS concentration was determined by UV/visible absorption spectroscopy with ε253 = 13,700 M−1·cm−1 (41). All calculated activation rates in this work refer to the amount of activatable Gt as determined by GTPγS titration.
Preparation of Bovine Rhodopsin and SEC.
Rod outer segment membranes were prepared as described in ref. 27. After incubation of the rod outer segment membranes (150 μM rhodopsin) with 5 μM 11-cis-retinal for 30 min at 4°C, DDM was added to a final concentration of 1.4% (wt/vol) followed by further incubation for 3 h. Unsolubilized material was removed by ultracentrifugation. Solubilized rhodopsin was purified by using the 1D4-immunoaffinity procedure described above (four wash steps). Then, 100 μl of purified rhodopsin was applied at a flow rate of 300 μl/min and 14–18°C to a Tricorn Superdex 200 300/10 GL column (GE Healthcare) equilibrated with buffer A containing 1 mM DTT and 0.03% (wt/vol) DDM by using a SMART system and SMART Manager software (Amersham Pharmacia/GE Healthcare). The same setup and buffer A containing 0.03% (wt/vol) DDM (with or without 1 mM DTT) was used for SEC of purified rhodopsin with Gt at different temperatures (4–12°C). Light activation of rhodopsin was performed as described below.
UV/Visible Spectroscopy.
Absorption spectra were taken at 20°C with a Cary 50 Bio UV/visible spectrophotometer (Varian, Palo Alto, CA) with a resolution of 1 or 2 nm. For each sample, absorption spectra between 250 and 650 nm were recorded in the dark and after illumination of the sample for 15 s with orange light with a 150-W fiber-optic light source equipped with a long-pass filter (GG 495; Schott, Elmsford, NY) and a heat-protection filter.
Molar Extinction Coefficients for Analysis of UV/Visible Absorption Data.
The following molar extinction coefficients were assumed: rhodopsin ε500 = 40,600 M−1·cm−1 (42), rhodopsin ε280 = 65,000 M−1·cm−1 (with an A280 nm/A500 nm ratio of ≈1.6), R* (metarhodopsin II) ε380 = 42,000 M−1·cm−1 (43), ε280 = 61,800 M−1·cm−1 [in metarhodopsin II, ε280 is ≈5% lower compared with rhodopsin (44)], Gt α-subunit ε280 = 30,400 M−1·cm−1, Gt βγ-subunit ε280 = 57,400 M−1·cm−1, and Gt (αβγ) ε280 = 87,800 M−1·cm−1. The values for Gt were calculated with Swiss-Prot entries GNAT1_BOVIN, GBB1_BOVIN, and GBG1_BOVIN with the ExPASy (www.expasy.org) ProtParam tool (45). The tool used the Edelhoch method (46) and extinction coefficients for Trp and Tyr determined by Pace et al. (47).
Calculation of the A280/A380 Ratio of the R*·Gt Complex.
For determination of the stoichiometry of R* and Gt in the R*·Gt complex from UV/visible absorption data obtained by SEC, the ratio of the absorption of the protein moiety in the R*·Gt complex to the retinal chromophore absorption was calculated as follows:
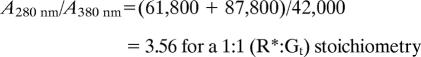 |
and
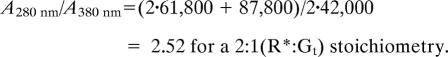 |
Calculation of GDP Released per R*·Gt Complex.
SEC runs with R*/Gt mixtures were performed with detection at 253 nm to monitor GDP (ε253 = 13,700 M−1·cm−1; ref. 41) and 380 nm to monitor R* (ε380 = 42,000 M−1·cm−1, see above). Release of one molecule of GDP per R*·Gt complex is given by the ratio of peak areas:
 |
and
 |
MALLS.
For mass determination, a combined setup consisting of SEC and subsequent online detection by UV absorption, three-angle static MALLS and differential refractive index measurement was used (48). SEC was performed with a Tricorn Superdex 200 10/300 GL column (GE Healthcare) connected to an ÄKTA purifier chromatography system (GE Healthcare) equilibrated with buffer A containing 1 mM DTT and 0.005 or 0.03% (wt/vol) DDM, respectively. For MALLS and differential refractive index measurements, a linear coupled miniDAWN Tristar system (Wyatt Technology, Santa Barbara, CA) and a differential refractive index detector (RI-101; Shodex, Kawasaki, Japan), respectively, were used. All calculations were performed with the software ASTRA (Wyatt Technology) on the basis of the detected signals for the UV absorption (280 nm), the refractive index (49), and three different scattering angles of scattered laser light. Experiments were performed at 4–6°C. Each experiment was repeated at least in triplicate. Under both detergent conditions of SEC, 0.005% or 0.03% (wt/vol) DDM, rhodopsin eluting in the SEC peak fraction was monomeric. For calculation of the molar masses, an extinction coefficient (at 280 nm) of 1,667 ml·g−1·cm−1 for rhodopsin and a dn/dc value of 0.133 for DDM (49) was used. Effects of interdetector band broadening between instruments in the online experiment were corrected by using ASTRA software.
Transducin Activation Assay.
As a monitor for Gt activation, changes in intrinsic fluorescence intensity of the Gα-subunit upon exchange of GDP to GTPγS were quantified (40). All measurements were carried out by using a SPEX fluorolog II spectrofluorometer equipped with a 450-W xenon arc lamp. For all activation measurements, settings were λex = 300 nm and λem = 345 nm with an integration time of 1 s. Gt activation rates were measured with 0–3 nM purified rhodopsin, 200 μM GTPγS, buffer A, 2 mM DTT, and 0.003–0.015% (wt/vol) DDM in a final volume of 80 μl (3 × 3 mm cuvette) or 650 μl (10 × 4 mm cuvette with stirring bar). All samples were equilibrated at 20°C for 4 min. Reactions were triggered with orange light (Schott GG 495 long-pass filter) after recording basic fluorescence levels for 50 s. After recording the activation at the respective R* concentration, activation of the whole Gt pool was achieved by adding 5 nM purified rhodopsin to the reaction. The maximal amplitude of fluorescence after addition of excess rhodopsin accounts for the total amount of Gt in the sample. Thus, the change in fluorescence (F/t) translates into activated Gt molecules per second. The whole set of experiments was repeated with different Gt and rhodopsin preparations.
The initial Gt activation rate (v) was obtained by linear regression of the initial rise in fluorescence emission. The fluorescence change was titrated with Gt, and the data points were fitted to a Michaelis–Menten type hyperbolic function: v = (Vmax·[Gαβγ])/(Km + [Gαβγ]),
where Vmax and Km denote the maximum value of v and the Michaelis constant, respectively, and [Gαβγ] is the effective Gt concentration. [Gαβγ] was calculated numerically in the fitting procedure by assuming that a fraction of the added Gt dissociates into its Gα and Gβγ subunits (with a dissociation constant K1) or binds to detergent micelles without rhodopsin (with a dissociation constant K2; see SI Methods). The data points of the titration experiments performed at three different DDM concentrations (0.01%, 0.008%, and 0.006%) were simultaneously fitted with the same set of parameters Vmax, K1, and K2 but with individual Michaelis constants (Km1, Km2, and Km3). DDM micelle concentration was set to 1.2 μM (0.006% DDM), 1.6 μM (0.008% DDM), and 2 μM (0.01% DDM).
FRET Experiments.
Fluorescence emission spectra between 450–600 nm (excitation at 420 nm) and 520–600 nm (excitation at 510 nm) of fluorescent proteins were recorded at 20°C with a SPEX fluorolog II spectrofluorometer and corrected for the fluorescence emission of the buffer. To determine FRET between mECFP and mVenus, the spectra of 11-cis-retinal-reconstituted purified R–mECFP and copurified R–mECFP/R–mVenus, respectively, were compared. Direct excitation of R–mVenus by the 420-nm excitation beam in the R–mECFP/R–mVenus mixture was accounted for as follows. (i) The emission spectrum of the R–mVenus reference sample (excitation at 420 nm) was scaled to the amount present in the R–mECFP/R–mVenus mixture by comparing maximal mVenus emission at 530 nm (excitation at 510 nm). (ii) The scaled R–mVenus emission spectrum (excitation at 420 nm) then was subtracted from the emission spectrum of the R–mECFP/R–mVenus mixture (excitation at 420 nm). The difference spectrum of the corrected R–mECFP/R–mVenus spectrum and the R–mECFP spectrum (matched to the peak height at 480 nm) yields the emission attributable to FRET. The same procedure was applied to analyze the mVenus–mECFP fusion protein. For the measurement, purified samples were diluted with 2 vol of buffer A containing 2 mM DTT, yielding a final concentration of 0.01% (wt/vol) DDM and 0.75 μM protein.
Supplementary Material
Acknowledgments
We thank Ingrid Semjonow for technical assistance and Arturo Zychlinsky and Cordula Enenkel for critically reading the manuscript. This work was supported by Deutsche Forschungsgemeinschaft Grants Sfb449 and Sfb740.
Abbreviations
- Gαβγ
heterotrimeric G protein
- Gt
transducin
- GPCR
G protein-coupled receptor
- R*
light-activated rhodopsin
- DDM
n-dodecyl-β-d-maltopyranoside
- SEC
size exclusion chromatography
- MALLS
multiangle laser light scattering
- mECFP
monomeric variants of enhanced cyan fluorescent protein.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701967104/DC1.
References
- 1.Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin JP, Davenport AP, Spedding M, Harmar AJ. Pharmacol Rev. 2005;57:279–288. doi: 10.1124/pr.57.2.5. [DOI] [PubMed] [Google Scholar]
- 2.Neves SR, Ram PT, Iyengar R. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 3.Liebman PA, Parker KR, Dratz EA. Annu Rev Physiol. 1987;49:765–791. doi: 10.1146/annurev.ph.49.030187.004001. [DOI] [PubMed] [Google Scholar]
- 4.Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K. Nature. 2003;421:127–128. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
- 5.Salom D, Lodowski DT, Stenkamp RE, Le Trong I, Golczak M, Jastrzebska B, Harris T, Ballesteros JA, Palczewski K. Proc Natl Acad Sci USA. 2006;103:16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George SR, O'Dowd BF, Lee SP. Nat Rev Drug Discov. 2002;1:808–820. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- 7.Terrillon S, Bouvier M. EMBO Rep. 2004;5:30–34. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milligan G. Mol Pharmacol. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- 9.Meyer BH, Segura JM, Martinez KL, Hovius R, George N, Johnsson K, Vogel H. Proc Natl Acad Sci USA. 2006;103:2138–2143. doi: 10.1073/pnas.0507686103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James JR, Oliveira MI, Carmo AM, Iaboni A, Davis SJ. Nat Methods. 2006;3:1001–1006. doi: 10.1038/nmeth978. [DOI] [PubMed] [Google Scholar]
- 11.Filipek S, Krzysko KA, Fotiadis D, Liang Y, Saperstein DA, Engel A, Palczewski K. Photochem Photobiol Sci. 2004;3:628–638. doi: 10.1039/b315661c. [DOI] [PubMed] [Google Scholar]
- 12.Filizola M, Wang SX, Weinstein H. J Comput Aided Mol Des. 2006;20:405–416. doi: 10.1007/s10822-006-9053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciarkowski J, Witt M, Slusarz R. J Mol Model. 2005;11:407–415. doi: 10.1007/s00894-005-0270-9. [DOI] [PubMed] [Google Scholar]
- 14.Mansoor SE, Palczewski K, Farrens DL. Proc Natl Acad Sci USA. 2006;103:3060–3065. doi: 10.1073/pnas.0511010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kota P, Reeves PJ, Rajbhandary UL, Khorana HG. Proc Natl Acad Sci USA. 2006;103:3054–3059. doi: 10.1073/pnas.0510982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Botelho AV, Huber T, Sakmar TP, Brown MF. Biophys J. 2006;91:4464–4477. doi: 10.1529/biophysj.106.082776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jastrzebska B, Maeda T, Zhu L, Fotiadis D, Filipek S, Engel A, Stenkamp RE, Palczewski K. J Biol Chem. 2004;279:54663–54675. doi: 10.1074/jbc.M408691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jastrzebska B, Fotiadis D, Jang GF, Stenkamp RE, Engel A, Palczewski K. J Biol Chem. 2006;281:11917–11922. doi: 10.1074/jbc.M600422200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filipek S, Stenkamp RE, Teller DC, Palczewski K. Annu Rev Physiol. 2003;65:851–879. doi: 10.1146/annurev.physiol.65.092101.142611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fotiadis D, Jastrzebska B, Philippsen A, Muller DJ, Palczewski K, Engel A. Curr Opin Struct Biol. 2006;16:252–259. doi: 10.1016/j.sbi.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Palczewski K. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chabre M, Cone R, Saibil H. Nature. 2003;426:30–31. doi: 10.1038/426030b. [DOI] [PubMed] [Google Scholar]
- 23.Chabre M, le Maire M. Biochemistry. 2005;44:9395–9403. doi: 10.1021/bi050720o. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann KP, Spahn CM, Heinrich R, Heinemann U. Trends Biochem Sci. 2006;31:497–508. doi: 10.1016/j.tibs.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Bigay J, Faurobert E, Franco M, Chabre M. Biochemistry. 1994;33:14081–14090. doi: 10.1021/bi00251a017. [DOI] [PubMed] [Google Scholar]
- 26.Berg OG, von Hippel PH. Annu Rev Biophys Biophys Chem. 1985;14:131–160. doi: 10.1146/annurev.bb.14.060185.001023. [DOI] [PubMed] [Google Scholar]
- 27.Heck M, Hofmann KP. J Biol Chem. 2001;276:10000–10009. doi: 10.1074/jbc.M009475200. [DOI] [PubMed] [Google Scholar]
- 28.Burns ME, Arshavsky VY. Neuron. 2005;48:387–401. doi: 10.1016/j.neuron.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Seitz HR, Heck M, Hofmann KP, Alt T, Pellaud J, Seelig A. Biochemistry. 1999;38:7950–7960. doi: 10.1021/bi990298+. [DOI] [PubMed] [Google Scholar]
- 30.Herrmann R, Heck M, Henklein P, Henklein P, Kleuss C, Hofmann KP, Ernst OP. J Biol Chem. 2004;279:24283–24290. doi: 10.1074/jbc.M311166200. [DOI] [PubMed] [Google Scholar]
- 31.Herrmann R, Heck M, Henklein P, Hofmann KP, Ernst OP. J Biol Chem. 2006;281:30234–30241. doi: 10.1074/jbc.M600797200. [DOI] [PubMed] [Google Scholar]
- 32.Fanelli F, Dell'Orco D. Biochemistry. 2005;44:14695–14700. doi: 10.1021/bi051537y. [DOI] [PubMed] [Google Scholar]
- 33.Dell'Orco D, Seeber M, Fanelli F. FEBS Lett. 2007;581:944–948. doi: 10.1016/j.febslet.2007.01.074. [DOI] [PubMed] [Google Scholar]
- 34.Fritze O, Filipek S, Kuksa V, Palczewski K, Hofmann KP, Ernst OP. Proc Natl Acad Sci USA. 2003;100:2290–2295. doi: 10.1073/pnas.0435715100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molday RS, MacKenzie D. Biochemistry. 1983;22:653–660. doi: 10.1021/bi00272a020. [DOI] [PubMed] [Google Scholar]
- 36.Griffin BA, Adams SR, Tsien RY. Science. 1998;281:269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- 37.Shaner NC, Steinbach PA, Tsien RY. Nat Methods. 2005;2:905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 38.Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. Nat Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- 39.Meyer CK, Böhme M, Ockenfels A, Gärtner W, Hofmann KP, Ernst OP. J Biol Chem. 2000;275:19713–19718. doi: 10.1074/jbc.M000603200. [DOI] [PubMed] [Google Scholar]
- 40.Ernst OP, Bieri C, Vogel H, Hofmann KP. Methods Enzymol. 2000;315:471–489. doi: 10.1016/s0076-6879(00)15862-8. [DOI] [PubMed] [Google Scholar]
- 41.Bock RM, Ling NS, Morell SA, Lipton SH. Arch Biochem Biophys. 1956;62:253–264. doi: 10.1016/0003-9861(56)90123-0. [DOI] [PubMed] [Google Scholar]
- 42.Wald G, Brown PK. J Gen Physiol. 1953;37:189–200. doi: 10.1085/jgp.37.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matthews RG, Hubbard R, Brown PK, Wald G. J Gen Physiol. 1963;47:215–240. doi: 10.1085/jgp.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin SW, Sakmar TP. Biochemistry. 1996;35:11149–11159. doi: 10.1021/bi960858u. [DOI] [PubMed] [Google Scholar]
- 45.Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. In: The Proteomics Protocols Handbook. Walker JM, editor. Totowa, NJ: Humana; 2005. pp. 571–607. [Google Scholar]
- 46.Edelhoch H. Biochemistry. 1967;6:1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- 47.Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wen J, Arakawa T, Philo JS. Anal Biochem. 1996;240:155–166. doi: 10.1006/abio.1996.0345. [DOI] [PubMed] [Google Scholar]
- 49.Strop P, Brunger AT. Protein Sci. 2005;14:2207–2211. doi: 10.1110/ps.051543805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.