Abstract
Nitric oxide (NO) is a widespread biological messenger that has many physiological and pathophysiological roles. Most of the physiological actions of NO are mediated through the activation of sGC (soluble guanylate cyclase) and the subsequent production of cGMP. NO also binds to the binuclear centre of COX (cytochrome c oxidase) and inhibits mitochondrial respiration in competition with oxygen and in a reversible manner. Although sGC is more sensitive to endogenous NO than COX at atmospheric oxygen tension, the more relevant question is which enzyme is more sensitive at physiological oxygen concentration. Using a system in which NO is generated inside the cells in a finely controlled manner, we determined cGMP accumulation by immunoassay and mitochondrial oxygen consumption by high-resolution respirometry at 30 μM oxygen. In the present paper, we report that the NO EC50 of sGC was approx. 2.9 nM, whereas that required to achieve IC50 of respiration was 141 nM (the basal oxygen consumption in the absence of NO was 14±0.8 pmol of O2/s per 106 cells). In accordance with this, the NO–cGMP signalling transduction pathway was activated at lower NO concentrations than the AMPKs (AMP-activated protein kinase) pathway. We conclude that sGC is approx. 50-fold more sensitive than cellular respiration to endogenous NO under our experimental conditions. The implications of these results for cell physiology are discussed.
Keywords: cytochrome c oxidase, high-resolution respirometry, mitochondrial respiration, nitric oxide (NO), oxygen consumption, soluble guanylate cyclase
Abbreviations: AMPK, AMP-activated protein kinase; 8-Br-cGMP, 8-bromoguanosine 3′,5′-cyclomonophosphate sodium salt; COX, cytochrome c oxidase; DEA NONOate, diethylamine NONOate (diazeniumdiolate) diethylammonium salt; DETA, diethylenetriamine; DMEM, Dulbecco's modified Eagle's medium; HBSS, Hanks balanced salt solution; HEK-293 cell, human embryonic kidney cell; IBMX, isobutylmethylxanthine; NO, nitric oxide; NOS, NO synthase; iNOS, inducible iNOS; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]-6-bromoquinoxazin-1-one; oxyHb, oxyhaemoglobin; PDE, phosphodiesterase; PKG, protein kinase G; PNPP, p-nitrophenyl phosphate; S-EITU, S-ethylisothiourea; sGC, soluble guanylate cyclase; Tet-iNOS 293, tetracycline-inducible iNOS-expressing HEK-293 cells; VASP, vasodilator-stimulated phosphoprotein
INTRODUCTION
NO (nitric oxide) is produced in cells by the enzyme NOS (NO synthase) from L-arginine, O2 and NADPH. Three isoforms of NOS are well characterized and referred to as nNOS (neuronal NOS; NOS-1), iNOS (inducible NOS; NOS-2) and eNOS (endothelial NOS; NOS-3). An mtNOS (mitochondrial isoform) has also been described, although its nature is controversial [1]. NO exerts a broad spectrum of functions in several systems, including the cardiovascular system, the central and peripheral nervous systems and the immune system. Two main cellular targets of NO have been clearly established. NO binds to the haem prosthetic group of sGC (soluble guanylate cyclase), stimulating the production of cGMP from GTP [2–5]. Through the binding to sGC, NO mediates many physiological effects such as smooth-muscle relaxation, inhibition of platelet aggregation and neurotransmission [6]. More recently, NO has also been shown to bind to and inhibit COX (cytochrome c oxidase) [7–11], the terminal enzyme of the mitochondrial electron-transport chain, which is responsible for almost all cellular O2 consumption. The inhibition is reversible and occurs in competition with O2. NO-mediated inhibition of O2 consumption has been reported in isolated COX [7], mitochondria [8,12,13], brain nerve terminals [7] and cultured cells [14,15]. High concentrations of NO have been shown to cause irreversible decreases in the affinity of COX for O2 [16]. The inhibition of respiration by NO could have pathological repercussions by limiting ATP production, but may also have a physiological function in controlling the rate of cellular O2 consumption and modulating the production of reactive O2 species by the respiratory chain [17].
The relative potency of NO towards these targets is a matter of debate. The EC50 of NO for sGC has been determined at 250 nM [18], 690 nM [19] or 1.6 μM [20] for the purified enzyme and 47 nM [21], 20 nM [22] or 2 nM [23] in cerebellar cells. The IC50 of NO on respiration at physiological O2 concentration has been reported as 60 nM in brain synaptosomes [7] and 11 nM in isolated brown adipose tissue mitochondria [13]. A major limitation of this kind of study is the difficulty in maintaining a constant NO supply for extended periods. Moreover, NO donors such as DETA (diethylenetriamine)-NO or SNAP (S-nitroso-N-acetyl-DL-penicillamine) are usually used as the NO source, despite their known limitations. More recently, it has been reported that sGC is significantly more sensitive to being activated by NO than COX is to being inhibited [24]. In that study, the EC50 of NO for sGC was 3.9 nM as determined in the purified enzyme, whereas the IC50 of NO for cellular respiration was determined as 120 nM in cerebellar cells at 30 μM O2. The source of NO used was DETA-NO and its concentration was kept constant by an ingenious system in which NO was continuously delivered by the donor and consumed by oxyHb (oxyhaemoglobin). However, it is not clear which enzyme is more sensitive to NO at physiological O2 tension when NO is produced inside the cells.
The purpose of the present study was to determine the relative sensitivity of the two main targets of NO in cells, sGC and mitochondrial respiration, to endogenous NO at physiological O2 tension (30 μM). To overcome difficulties associated with the use of NO donors to generate NO, we used a HEK-293 (human embryonic kidney) cell line transfected with the iNOS gene under a tetracycline-inducible promoter [25]. By adding tetracycline to the incubation medium we were able to induce iNOS expression to different levels and thus to produce NO at controlled levels for extended periods. Using this system we have monitored NO generation with electrochemical NO electrodes and the cGMP production by immunoassay inside hypoxic chambers to maintain a constant O2 tension of 30 μM. We have also measured NO-mediated inhibition of respiration by high-resolution respirometry. Because the inhibition of COX by NO depends on the O2 tension, we measured respiration inhibition at physiological O2 concentration (30 μM). Finally, we have compared the NO-sensitivities of the PKG (protein kinase G; also known as cGMP-dependent protein kinase) and AMPK (AMP-activated protein kinase) signal transduction pathways. Our results show that the EC50 of NO for sGC is approx. 50-times lower than the IC50 of NO on cellular respiration at 30 μM O2. We also show that the NO–cGMP signalling pathway is activated at lower NO concentrations than the AMPK pathway. We conclude that sGC is a more sensitive target of endogenous NO than cellular respiration at physiological O2 concentration.
EXPERIMENTAL
Cell culture and reagents
Tetracycline-inducible HEK-293 cells stably expressing human iNOS [Tet-iNOS 293 (tetracycline-inducible iNOS-expressing HEK-293 cells)] were generated as described elsewhere [25]. Cells were cultured in DMEM (Dulbecco's modified Eagle's medium; Gibco, Invitrogen, Barcelona, Spain) containing 4.5 g/l D-glucose, 10% (v/v) foetal calf serum, 200 μg/ml hygromycin B and 15 μg/ml blasticidin, in the dark at 37°C in a humidified atmosphere with 5% CO2. S-EITU (S-ethylisothiourea) hydrobromide, L-arginine, 8-Br-cGMP (8-bromoguanosine 3′,5′-cyclomonophosphate sodium salt), ODQ (1H-[1,2,4]oxadiazolo[4,3-a]-6-bromoquinoxazin-1-one), IBMX (isobutylmethylxanthine) and DEA NONOate [diethylamine NONOate (diazeniumdiolate) diethylammonium salt] were from Sigma–Aldrich (St. Louis, MO, U.S.A.). Hygromycin B and blasticidin were from Invitrogen. Tetracycline was from Calbiochem (Darmstadt, Germany).
Induction of endogenous NO production
iNOS expression was induced by overnight incubation of cells in complete growth medium (without selection antibiotics) containing tetracycline (2–1000 ng/ml) and 500 μM S-EITU, a potent inhibitor of iNOS activity. Cells were washed with L-arginine-free DMEM (Invitrogen) supplemented with 1% foetal calf serum dialysed to avoid any traces of L-arginine that could trigger undesired NO production. Cells were incubated in L-arginine-free medium for 1 h to completely eliminate S-EITU and tetracycline without activating NO production. Cells were trypsinized and resuspended at 1×107 cells/ml in HBSS (Hanks balanced salt solution) containing 25 mM Hepes. Endogenous NO production was triggered by adding 1 mM L-arginine (see below).
Immunoblot analysis of iNOS, VASP (vasodilator-stimulated phosphoprotein) and phospho-AMPK
Total protein extracts were prepared as follows: for iNOS analysis, attached cells were harvested by trypsinization and resuspended in HBSS at 1×107 cells/ml. For VASP and phospho-AMPK analysis, attached cells were scraped off in 1.5 ml of ice-cold PBS freshly supplemented with phosphatase inhibitors [6 mM NaF, 10 mM 2-glycerophosphate, 10 mM PNPP (p-nitrophenyl phosphate) and 1 mM NaVO3] and a protease inhibitor cocktail (Roche Diagnostics, Barcelona, Spain). Cells were centrifuged at 300 g for 5 min at 4°C and the pellet was resuspended in 60 μl of ice-cold lysis buffer [20 mM Hepes, pH 7.5, 400 mM NaCl, 20% (v/v) glycerol, 0.1 mM EDTA, 10 mM NaF, 10 μM Na2MoO4, 1 mM NaVO3, 10 mM PNPP and 10 mM 2-glycerophosphate] supplemented with 1 mM dithiothreitol, 1 mM Pefablock® (pH 7.4) and a tablet of protease inhibitor cocktail. Cells were lysed on ice for 15 min, and cleared whole-cell extracts were obtained by recovering the supernatant after centrifugation (16000 g for 15 min at 4°C). After protein determination (see below), an equal amount of each protein sample (200 μg for iNOS and 120 μg for VASP and phospho-AMPK) was resolved by SDS/7.5% (w/v) PAGE and transferred on to nitrocellulose membranes (Amersham Biosciences, Little Chalfont, Bucks., U.K.) by using standard procedures [26]. Thereafter, membranes were blocked with 5% (w/v) non-fat dried milk in TBS-T (20 mM Tris/HCl, pH 7.2, 150 mM NaCl and 0.1% Tween 20) and incubated overnight with a polyclonal antibody against iNOS (1:2000; Transduction Laboratories, BD Biosciences, Erembodegem, Belgium), a polyclonal antibody against human VASP (1:1000; Calbiochem, San Diego, CA, U.S.A.), a polyclonal anti-phospho-AMPKα (Thr172) antibody (1:1000; Cell Signaling Technology, Beverly, MA, U.S.A.) or a monoclonal anti-actin antibody (1:10000; Sigma–Aldrich) in blocking solution at 4°C. Protein bands were detected by incubation for 1 h with horseradish peroxidase-coupled goat anti-rabbit IgG (1:5000; Vector Laboratories, Burlingame, CA, U.S.A.) or goat anti-mouse IgG (1:2500; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) in blocking solution at room temperature, followed by enhanced chemiluminescence (ECL®; Amersham Biosciences).
Quantification of NO
Endogenous NO production was determined in cells suspended at 1×107 cells/ml in HBSS containing 25 mM Hepes. Measurements were taken with an NO electrode (ISO-NOP; World Precision Instruments, Stevenage, Herts., U.K.) in 1 ml of cell suspension in gas-tight chambers gently agitated and kept at 37°C. The NO electrode was calibrated by addition of known concentrations of NaNO2 under reducing conditions (KI/H2SO4) at 37°C. Calibrations were performed at low O2 tension (30 μM) to take account of the impact of the autoxidation reaction of NO. To initiate NO production, 1 mM L-arginine was added to the chamber at 30 μM O2 measured with an oxygen electrode (Hansatech, King's Lynn, Norfolk, U.K.). The oxygen electrode was calibrated with air-saturated incubation medium at 37°C, assuming an atmospheric O2 concentration of 200 μM. NO production was monitored for at least 10 min after the addition of L-arginine.
Measurement of oxygen consumption
O2 consumption at physiological tissue O2 concentration (30 μM) was determined by high-resolution respirometry using an Oroboros Oxygraph-2k instrument. Cells were trypsinized after overnight treatment with tetracycline and resuspended at 1×107 cells/ml in HBSS containing 25 mM Hepes. The instrumental background flux was calculated as a linear function of O2 concentration and the experimental data were corrected for the whole range of O2 concentrations using DatLab software (Oroboros Instruments). The O2 concentration in the culture medium at air saturation at 37°C and local barometric pressure (92.6 kPa) was 175.7 μM (O2 solubility factor 0.92). Measurements were taken at 37°C in parallel Oxygraph-2k chambers for cells treated with the same amount of tetracycline; NO production was initiated in one chamber by the addition of L-arginine (1 mM). Because light has been reported to reverse the inhibition of respiration induced by NO [27], all experiments were performed in the absence of direct light. NO was quenched by the addition of oxyHb to study the reversibility of respiration inhibition.
Measurement of cGMP
For the measurement of cGMP, cells were plated on to six-well plates at 1.5×106 cells/well. After reaching confluence, cells were incubated with tetracycline overnight to induce iNOS expression as described above. Plates were transferred to hypoxic chambers (Coy Laboratory Products, Grass Lake, MI, U.S.A.) at 37°C and set at an O2 concentration of 30 or 15 μM. The medium was replaced with HBSS supplemented with 1 mM L-arginine to initiate NO production, and incubations were carried out for different periods up to 5 min. Afterwards, 0.1 M HCl was added for 30 min to stop endogenous PDE (phosphodiesterase) activity and to lyse the cells. Lysates were scraped off the plates and centrifuged at 1200 g for 5 min at room temperature, and the supernatant was used directly in the assay. cGMP was measured by immunoassay using the cGMP EIA Kit for Cell and Tissue Lysates (Biomol, Exeter, U.K.) according to the manufacturer's instructions. Protein concentration was determined in the same supernatants used to quantify cGMP.
Protein measurement
Protein concentration was measured with the BCA (bicinchoninic acid) protein assay kit (Pierce; from Perbio Science, Tattenhall, Cheshire, U.K.) using BSA as the standard.
RESULTS AND DISCUSSION
Production of endogenous NO by Tet-iNOS 293 cells
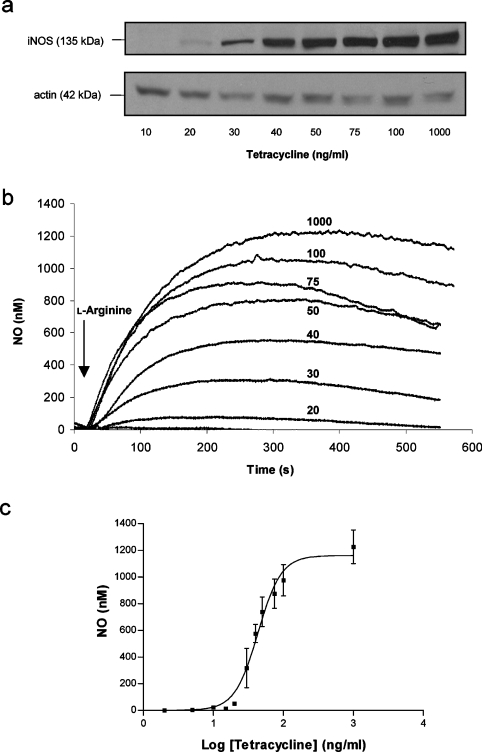
Overnight incubation of cells with a range of concentrations of tetracycline (2–1000 ng/ml) led to a concentration-dependent expression of iNOS protein as determined by Western blotting (Figure 1a). The addition to the cells of the iNOS substrate L-arginine (1 mM) at 30 μM O2 immediately triggered NO production (Figure 1b). The quantity of NO produced was dependent on the amount of iNOS expressed and remained constant for at least 10 min. The highest NO concentration obtained, at the greatest expression of iNOS, was 1.2±0.1 μM (n=3). NO concentration returned to basal after the addition of oxyHb (Figure 5d). The relationship between tetracycline concentration and the maximum value of endogenous NO production at peak time is shown in Figure 1(c). The NO concentrations produced by our cells are within the reported physiological range, which appears to be between 1 nM and 1 μM, depending on the tissue and conditions [28]. Moreover, many cell types will express iNOS and produce high levels of NO when activated by inflammatory mediators, for example endotoxin and interferon γ. Indeed, activated astrocytes accumulate up to 1 μM NO in the medium, with cellular O2 consumption approximately half that of control cells [15]. In the absence of L-arginine, iNOS is able to produce superoxide mainly at the flavin-binding sites of the reductase domain [29], although at lower levels than the neuronal isoenzyme [30].
Figure 1. Expression of iNOS and production of endogenous NO.
(a) Immunoblot analysis showing the expression of iNOS after overnight induction of cells with a range of tetracycline concentrations. Immunoblot with anti-actin antibody confirmed equal protein loading. (b) Online electrochemical detection of NO gas production in cells treated with tetracycline. NO production was detected immediately after the addition of L-arginine (1 mM) to the chamber of an O2 electrode containing 1×107 cells in suspension at 37°C. Addition of L-arginine was performed at 30 μM O2 and NO was monitored for at least 10 min. (c) Relationship between the tetracycline concentration used to induce iNOS expression overnight and maximal NO production after L-arginine addition at 30 μM O2. The results presented in (a, b) are representative of results obtained in three separate experiments. Results in (c) are the means and S.D. for at least three independent experiments.
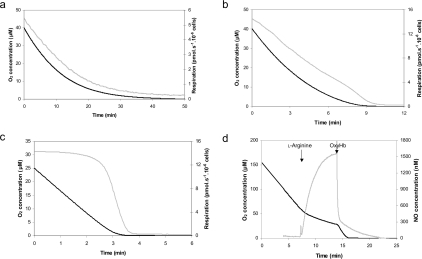
Figure 5. Inhibition of respiration induced by endogenous NO.
Online recording of O2 concentration (black line) and O2 flux (grey line) determined by high-resolution respirometry in cells pre-treated overnight with (a) 50, (b) 20 or (c) 2 ng/ml tetracycline and then stimulated by addition of L-arginine (1 mM) at an O2 concentration of 60 μM (a, b) or 30 μM (c). The low range of O2 concentrations [≤40 μM (a, b) and ≤25 μM (c)] and the corresponding O2 flux are shown as a function of time. (d) Online simultaneous recording of O2 concentration (black line) and NO gas production (grey line) in cells treated with tetracycline (1000 ng/ml). NO production started right after L-arginine addition (1 mM) and immediately inhibited respiration. Addition of oxyHb quenched NO and restored the control respiration rate. The results presented in each panel are representative of results obtained in at least three separate experiments.
Sensitivity of sGC to endogenous NO
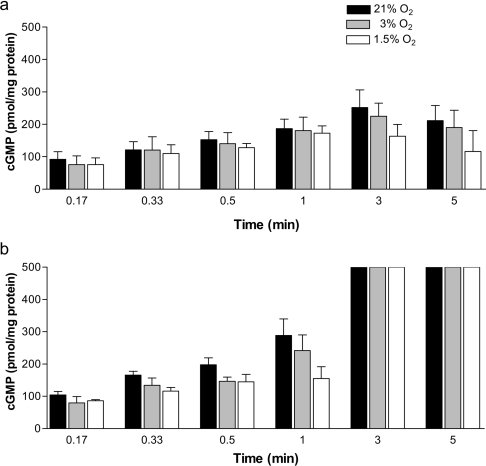
sGC is a key NO receptor protein involved in NO-mediated biological and pathological reactions. NO activates sGC, leading to increased production of the intracellular messenger cGMP, whose biological effects are mediated by PKGs, cGMP-regulated ion channels and cGMP-dependent PDEs [31]. To examine the kinetics of NO-induced sGC activation in our system, cells were incubated with tetracycline (5 ng/ml) overnight and then induced with L-arginine (1 mM) to produce NO for periods up to 5 min. We chose 5 ng/ml tetracycline for the time course experiment, despite the fact that at this concentration NO production was below the detection limit of our electrode, because this tetracycline concentration yielded a clear increase in cGMP production with time. cGMP accumulation was determined by immunoassay in hypoxic chambers at three different O2 concentrations: 200, 30 and 15 μM, which correspond to 21, 3 and 1.5% O2 respectively (Figure 2). cGMP began to accumulate immediately after L-arginine addition, reaching a maximum at 3 min and decreasing slightly at 5 min (Figure 2a). The NO produced upon addition of L-arginine (Figure 1b) activates sGC to generate cGMP from GTP. The amounts of cGMP observed reflect a balance between cGMP production and degradation. cGMP in cells is degraded by PDEs, which also determine the shape of the cGMP signal [32]. The activity of endogenous PDEs is different in different cell types; for example, PDE activity is lower in cerebellar cells than in striatal cells [33]. It has been reported that exposure of cells to DEA NONOate for 2 min desensitizes sGC and that the degree of desensitization is related to the cGMP concentration [22,33]. This could explain the decrease in cGMP levels observed at 5 min, particularly considering that our standard assay medium used to determine cGMP contains no PDE inhibitor that could lead to artificially high cGMP levels. Addition of 0.5 mM IBMX, an inhibitor of endogenous PDEs, increased the maximum levels of cGMP in the cell extracts saturating our assay from 3 min onwards, and completely preventing the decrease in cGMP at prolonged incubations (Figure 2b).
Figure 2. Time course of endogenous NO-induced cGMP production at three O2 concentrations.
(a) Cells were treated overnight with tetracycline (5 ng/ml), and a time course of NO-activated cGMP production was measured inside hypoxic chambers at three O2 concentrations (21, 3 and 1.5% O2). Maximal cGMP accumulation was detected 3 min after L-arginine (1 mM) administration. (b) Time course as in (a), but in the presence of IBMX (0.5 mM), an inhibitor of endogenous PDEs. Values are the means and S.D. for at least three independent experiments.
To study the relationship between O2 concentration and sGC activity, we performed the experiments at three different O2 concentrations. The accumulation of cGMP was essentially independent of the O2 tension, although at very low tension (1.5% O2) a slight decrease in cGMP levels was observed (Figure 2a). Effects of O2 tension on the expression of NOS have been extensively studied in vitro [34,35] and in vivo [36–38]. Moreover, it has been reported that sGC function is impaired in pulmonary arteries of rats exposed to chronic hypoxia [39] and that hypoxia decreases sGC expression in pulmonary artery smooth-muscle cells [40]; however, it had been previously shown that sGC expression increases in lungs from rats exposed to hypoxia [41]. In our experiments, cells were exposed to hypoxia only after overnight incubation with tetracycline, during the 1 h incubation in L-arginine-free medium and the subsequent 3 min treatment with L-arginine. We therefore believe that the slight decrease in cGMP production at low O2 tension is not related to decreased expression levels of the protein, but could instead reflect the fact that NOS activity is O2-dependent and that the Km of NOS for O2 is high [42]. Supporting this view is the fact that cGMP accumulation at 3 and 1.5% O2 was similar in Tet-iNOS 293 cells treated with DEA NONOate (1 μM), from which the release of NO is O2-independent (results not shown).
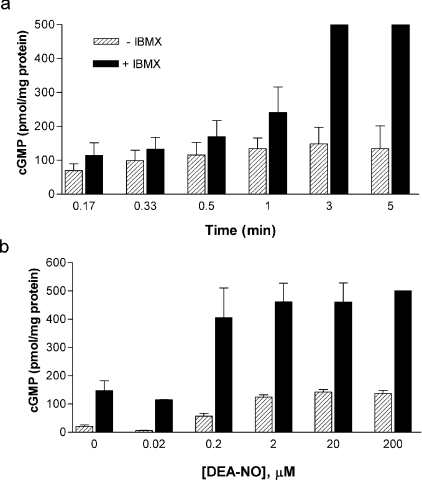
To further correlate the release of NO with cGMP effects, we conducted a time course experiment of cGMP production triggered by DEA NONOate, which is a fast-release NO donor (2.5 min half-life at neutral pH and 37°C; Figure 4a). The NO concentration released by 1 μM DEA NONOate in medium containing 1×107 cells/ml at 37°C was 268±56 nM (n=6). At 3% O2, the pattern of cGMP production after the addition of 1 μM DEA NONOate was similar to that found with endogenous NO produced by tetracycline-induced iNOS: a time-dependent increase in cGMP accumulation, which was highest at 3 min and slightly decreased at 5 min (Figure 4a). Again, in the presence of 0.5 mM IBMX, the cGMP concentration increased significantly, saturating our assay at 3 and 5 min (Figure 4a).
Figure 4. DEA NONOate-induced cGMP production at 30 μM O2.
(a) Time course of cGMP production in cells treated with DEA NONOate (1 μM) in the absence or presence of IBMX (0.5 mM) at 30 μM O2. Maximal cGMP accumulation was detected at 3 min of incubation. IBMX significantly increased the cGMP accumulation at 3 and 5 min, saturating our assay. (b) Dose response of cGMP production induced by treatment of cells with DEA NONOate (0.02–200 μM) for 3 min at 30 μM O2 in the absence or presence of IBMX (0.5 mM). Values are the means ± S.D. for three independent experiments.
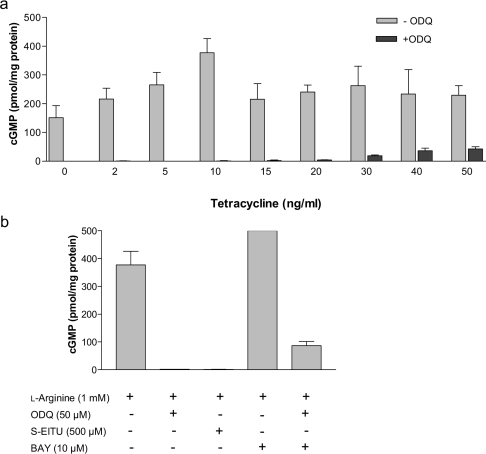
To study the sensitivity of sGC to endogenous NO, cells were incubated overnight with a range of tetracycline concentrations (2–50 ng/ml) and then induced with L-arginine (1 mM) to produce NO for 3 min at physiological O2 tension (30 μM; Figure 3a). The incubation time of 3 min was selected because this coincided with peak cGMP accumulation in the previous experiment (Figure 2a). cGMP production increased in a dose-dependent manner up to a maximum value of 380 pmol/mg of protein at 10 ng/ml tetracycline and then decreased to approx. 230 pmol/mg of protein regardless of the tetracycline concentration used. Interestingly, at high tetracycline doses (>50 ng/ml), NO activated sGC and cGMP production, even in the absence of exogenously added L-arginine (results not shown). The activity of endogenous iNOS normally requires extracellular arginine, in spite of the fact that intracellular arginine concentrations are sufficient to saturate the enzyme. The explanation reported for this is that arginine uptake is required to de-repress translation of iNOS [43]. In our system, however, the tetracycline-induced iNOS expression is not subject to this regulation, and low concentrations of intracellular arginine are therefore able to stimulate NO synthesis when iNOS expression is high and this, in turn, activates sGC to produce cGMP.
Figure 3. Endogenous NO-induced cGMP production at 30 μM O2.
(a) Cells were treated overnight with a range of tetracycline concentrations (2–50 ng/ml), and NO-activated cGMP production was determined 3 min after the addition of L-arginine (1 mM) inside hypoxic chambers at 30 μM O2 in the absence and presence of ODQ (50 μM), a potent and selective inhibitor of NO-sensitive sGC. (b) cGMP production in 10 ng/ml tetracycline-treated cells exposed for 3 min to L-arginine (1 mM) or to L-arginine plus the activator BAY 41-2272 (10 μM) at 30 μM O2. Addition of ODQ (50 μM) or S-EITU (500 μM) during the 3 min incubation period prevented cGMP accumulation. Values are the means ± S.D. for at least three independent experiments.
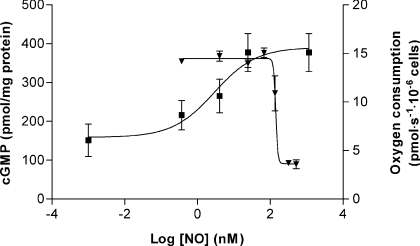
The accumulation of cGMP observed after overnight incubation of the cells with tetracycline and induction with L-arginine (1 mM) was partially prevented by the addition to the incubation medium (3 min incubation) of inhibitors of sGC (50 μM ODQ; Figures 3a and 3b) and totally prevented by iNOS inhibitors (500 μM SEITU; Figure 3b). ODQ completely inhibited cGMP production at low doses of tetracycline but this effect was only partial at high tetracycline doses (Figure 3a) which is consistent with the competitive nature of the inhibition [44]. These experiments show that the observed increase in cGMP can be attributed to the specific activation of sGC by NO. We used HEK-293 cells transfected with empty vector (not containing the iNOS gene) as a negative control. In these cells, overnight treatment with tetracycline and addition of L-arginine did not produce any detectable accumul-ation of cGMP (results not shown). As a positive control, BAY 41-2272 {5-cyclopropyl-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-4-ylamine} (10 μM), an NO-independent but haem-dependent sGC activator [45], stimulated cGMP production to levels that saturated our assay (>500 pmol/mg of protein; Figure 3b). Figure 3(b) also shows that the activation of sGC by BAY 41-2272 was partially prevented by ODQ (50 μM). Figure 6 shows the relationship between the concentration of NO produced endogenously and the amount of cGMP produced by sGC at 3 min, 37°C and 30 μM O2. The NO concentration that induced EC50 of the enzyme was estimated as 2.9 nM.
Figure 6. NO concentration–response curves of cGMP production and inhibition of respiration at 30 μM O2.
The NO concentration that induces EC50 of sGC was estimated as 2.9 nM. The IC50 value of NO for cellular respiration was determined to be 141 nM. Values are the means ± S.D. for at least three independent experiments.
To our surprise, concentrations of tetracycline above 10 ng/ml did not yield additional increases in cGMP. To try to explain this result, we performed a dose–response experiment of cGMP production in cells incubated for 3 min with DEA NONOate with or without IBMX (Figure 4b). Again, widely differing DEA NONOate concentrations (2, 20 and 200 μM), which produce very different amounts of NO in medium containing 1×107 cells/ml at 37°C (918±79 nM, 9217±928 nM, n=5, and >9 μM respectively) did not yield significantly different values of cGMP accumulation at 3 min (Figure 4b). The addition of IBMX (0.5 mM) increased cGMP to almost saturate our assay at DEA NONOate concentrations of 2 μM and above.
In light of the DEA NONOate experiments it seems likely that, regardless of the NO source, NO concentrations above a certain threshold do not produce additional increases in cGMP accumulation. In other words, sGC becomes insensitive to NO above a particular level or time of exposure. This is supported by the reported desensitization of sGC to DEA NONOate and the fact that the degree of desensitization is related to the cGMP concentration [22,33]. The combination of PDE activity and sGC desensitization could account for the plateau observed at tetracycline concentrations higher than 10 ng/ml.
Sensitivity of mitochondrial respiration to endogenous NO
The mean capillary O2 concentration is approx. 30 μM and the typical value inside cells averages 3 μM [46]. These low tissue O2 tensions do not limit mitochondrial respiration, since the affinity of COX for O2 is very high, and so there is a very large safety margin to sustain mitochondrial oxidative phosphorylation [47,48]. Nevertheless, at these O2 tensions, NO might compete significantly for COX binding, and so could inhibit respiration. NO and other inhibitors that raise the enzyme's Km, will decrease the affinity of COX for O2 and thereby induce O2 limitation under apparently normoxic conditions. Moreover, biochemical and physiological studies suggest that Hb possesses an allosterically regulated nitrite reductase activity that reduces nitrite to NO along the physiological O2 gradient [49]. To study the sensitivity of mitochondrial respiration to endogenous NO, we determined O2 concentration and O2 flux by high-resolution respirometry in Tet-iNOS 293 cells treated with tetracycline (2–50 ng/ml; Table 1). High-resolution respirometry provides a means to accurately determine respiration at low O2 tension [50]. In these experiments, L-arginine (1 mM) was added to cells at 60 μM O2. As shown in Figure 5, in cells treated overnight with a high tetracycline dose (50 ng/ml), cellular O2 consumption was significantly inhibited immediately after the addition of L-arginine (Figure 5a). The control value in the absence of NO was 14±0.8 pmol of O2/s per 106 cells. Since the inhibition of COX by NO is competitive with O2, NO is a more effective inhibitor at low O2 tensions and hence the degree of inhibition of respiration is dependent on the O2 concentration. For example, in cells stimulated with the highest tetracycline concentration used in these experiments (50 ng/ml), the inhibition of respiration from the control value was 49% at 50 μM O2 and 67% at 30 μM O2. The addition of oxyHb to completely quench NO, fully re-activated cellular respiration (Figure 5d), which shows that the inhibitory effect is reversible. The lowest tetracycline concentration that had an effect on respiration at low O2 was 20 ng/ml (Figure 5b). At physiological O2 tension (30 μM) the concentration of NO that caused an IC50 of cellular respiration was 141 nM (Figure 6).
Table 1. Respiration rate at physiological O2 (30 μM) and increasing NO concentrations.
Respiration rate and NO production at 30 μM O2 in cells treated overnight with tetracycline (2–50 ng/ml) and activated by addition of L-arginine (1 mM). Measurements of O2 concentration and O2 flux in control and NO-producing cells (1×107 cells/ml) were taken in parallel chambers of a high-resolution respirometer (Oroboros Oxygraph-2k) at 37°C as explained in the Experimental section. NO production was initiated by addition of L-arginine at 60 μM O2. Values are the means ± S.D. for three independent experiments.
| Tetracycline (ng/ml) | NO concentration (nM) | Respiration at 30 μM O2 (pmol of O2/s per 106 cells) |
|---|---|---|
| 2 | n.d.* | 14.2±0.17 |
| 5 | n.d. | 14.7±0.53 |
| 10 | n.d. | 13.9±0.38 |
| 15 | 14.5±9.08 | 15.1±0.50 |
| 20 | 52.4±9.34 | 10.9±1.80 |
| 30 | 318±128 | 3.69±0.05 |
| 40 | 576±59.8 | 3.58±0.45 |
| 50 | 739±96.9 | 4.62±0.16 |
* n.d., Not detectable.
We performed additional measurements of respiration and NO in cells treated with low tetracycline concentrations (2, 5 and 10 ng/ml). For respiration measurement, L-arginine was added at 30 μM O2 instead of 60 μM; NO was not detectable in any case. We measured the respiration at 15 μM O2 (1.5% O2). The lack of effect of NO produced by cells treated with 2 ng/ml tetracycline on respiration is shown in Figure 5(c). Table 2 shows the values of respiration at 15 μM O2 as a percentage of the value at 40 μM (before arginine addition) in cells treated with 2, 5 and 10 ng/ml tetracycline. The results indicate that there are no significant differences in any case.
Table 2. Respiration rate at low O2 (15 μM) and low NO concentrations.
Respiration rate at 15 μM O2 expressed as a percentage of respiration at 40 μM O2 (before L-arginine addition) in cells treated with low doses of tetracycline (2, 5 and 10 ng/ml). Measurements of O2 concentration and O2 flux in control and NO-producing cells (1×107 cells/ml) were taken in parallel chambers of a high-resolution respirometer (Oroboros Oxygraph-2k) at 37°C as explained in the Experimental section. NO production was initiated by the addition of L-arginine (1 mM) at 30 μM O2. Values are the means ± S.D. for three independent experiments.
| Tetracycline (ng/ml) | L-Arginine (1 mM) | Respiration at 15 μM (% of respiration at 40 μM) |
|---|---|---|
| 2 | + | 98.4±1.7 |
| – | 99.8±1.2 | |
| 5 | + | 96.6±3.0 |
| – | 99.7±0.5 | |
| 10 | + | 97.1±0.8 |
| – | 98.2±1.4 |
The rate of cellular O2 consumption is controlled by a number of factors [51,52]. The excess capacity of COX explains the low control coefficient of the enzyme over respiration. Therefore, a fall in COX activity will not have a proportional effect on the cellular O2 consumption rate. In other words, COX activity may be partially inhibited without any effect on mitochondrial respiration, so although low NO concentrations had no effect on the respiration rate of our cells, an actual decrease in COX activity cannot be ruled out. However, there is a threshold value beyond which respiration will be inhibited. In this respect, it is important to point out a recent study in respiring cells showing that, as the O2 concentration decreases, there is an early reduction of cytochromes aa3 and cc1, part of the COX enzyme, relative to the decrease in the rate of O2 consumption [53], a mechanism in which NO might be involved and that has important signalling consequences.
Consequences of NO effects for cell signalling
To further study the relative sensitivities of the two main cellular targets of NO, and considering the fact that COX activity can be partially inhibited without an effect on respiration, we examined the effects of NO on cell signalling through PKG and AMPK (Figure 7).
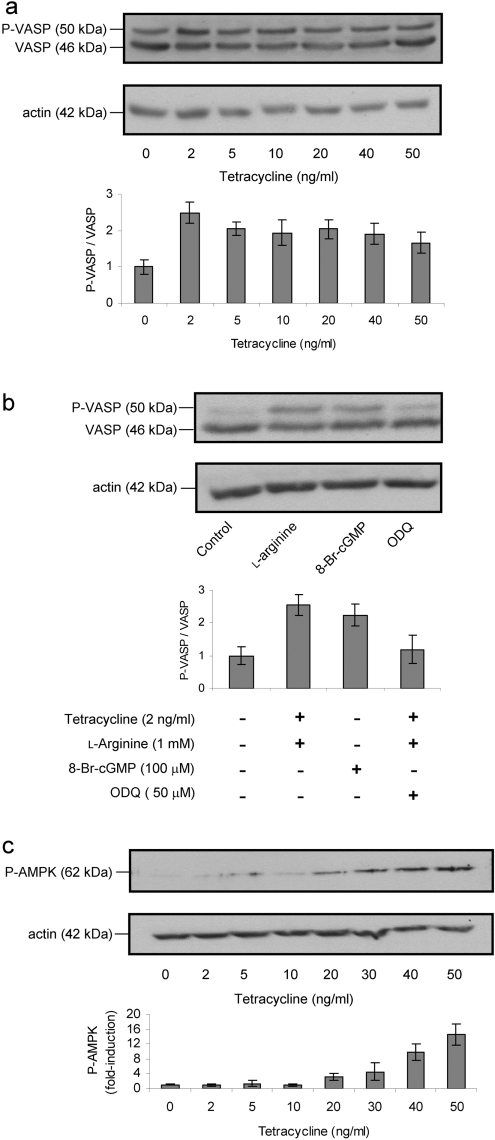
Figure 7. Phosphorylation of VASP and AMPK induced by endogenous NO.
(a) Immunoblot analysis of non-phosphorylated VASP and VASP phosphorylated at Ser157 (P-VASP) after overnight induction of the cells with a range of tetracycline concentrations. NO production was induced by addition of L-arginine (1 mM) 1 min before cell lysis. The anti-VASP antibody detects two bands, the upper band corresponding to P-VASP. Immunoblot densitometric results are presented as the mean and range of P-VASP/VASP ratio. (b) Exposure for 1 min to 8-Br-cGMP (100 μM) induced VASP phosphorylation in control cells (not treated with tetracycline), whereas the addition of ODQ (50 μM) to cells incubated with tetracycline (5 ng/ml) plus 1 min exposure to L-arginine (1 mM) prevented NO-induced VASP phosphorylation. (c) Immunoblot analysis showing the expression of phosphorylated AMPK (P-AMPK) after overnight induction of the cells with a range of tetracycline concentrations. NO production was induced by addition of L-arginine (1 mM) 15 min before cell lysis. The results presented in (a–c) are representative of results obtained in two separate experiments. Immunoblots with anti-actin antibody confirmed equal protein loading.
PKG is a serine/threonine protein kinase and is one of the major intracellular receptors for cGMP. Activated PKG phosphorylates many intracellular proteins and regulates important physiological functions such as relaxation of vascular smooth muscle, inhibition of cell differentiation and proliferation and inhibition of platelet aggregation and apoptosis [31]. We measured the phosphorylation state of the PKG substrate VASP, which is expressed in most mammalian tissues. Phosphorylation of VASP at Ser157 causes a mobility shift on SDS/PAGE from 46 to 50 kDa, providing a convenient marker of PKG activity. The antibody that we used in the present study recognizes Ser157-phosphorylated VASP and the non-phosphorylated form. VASP phosphorylation has been established as a marker of the activity of the NO–cGMP pathway in various models [54], including HEK-293 cells [55].
AMPK plays a key role in the regulation of energy homoeostasis and is activated by an elevated AMP/ATP ratio produced in response to cellular and environmental stresses, such as heat shock, hypoxia and ischaemia [56]. AMPK is itself phosphorylated by the upstream component of the protein kinase cascade AMPKK (AMPK kinase) and this phosphorylation is required for AMPK activation [57]. The phospho-AMPKα (Thr172) antibody (Cell Signaling Technology) used in the present study detects endogenous AMPKα only when phosphorylated at Thr172, and thus acts as a marker of AMPK activity.
Time course experiments indicated that NO-induced phosphorylation was maximal at 1 min for VASP and 15 min for AMPK, as determined by immunoblot (results not shown). The increase in VASP phosphorylation was transient (decreasing after 1 min), whereas AMPK phosphorylation was maintained for up to 1 h. The dose–response experiment for VASP phosphorylation by NO produced a maximal phosphorylated VASP/VASP ratio at 2 ng/ml tetracycline, with lower values at higher concentrations (Figure 7a). 8-Br-cGMP, a membrane-permeable cGMP derivative, induced VASP phosphorylation in the absence of NO, and ODQ, an inhibitor of sGC, confirmed that VASP phosphorylation is dependent on sGC activation (Figure 7b). The increase in AMPK phosphorylation was maximal at 20 ng/ml tetracycline and was maintained at higher NO concentrations (Figure 7c). These experiments agree with the results presented earlier in the present study in indicating that the NO–PKG signalling pathway is activated at lower NO concentrations than the NO–AMPK pathway.
In conclusion, our results show that the IC50 for respiratory inhibition by NO is approx. 50-fold higher than the EC50 for activation of sGC by NO at tissue O2 levels. Our results suggest that it is unlikely that NO concentrations that maximally activate sGC could simultaneously inhibit cellular respiration. This agrees with the greater sensitivity of isolated sGC to exogenous NO (DETA-NO) in comparison with mitochondrial respiration [24]. Moreover, the NO concentrations that activate the NO–cGMP signalling pathway are lower than those required to activate AMPK. However, given that intracellular O2 concentrations are an order of magnitude lower than tissue O2 levels, it is possible that in this lower range of O2 concentrations, NO regulates cell respiration as well as cGMP production during normal physiology or under pathological conditions of ischaemia and hypoxia.
Acknowledgments
We are grateful to Salvador Moncada (Wolfson Institute for Biomedical Research, London, U.K.) for helpful comments on this paper and for kindly supplying BAY 41-2272. We also thank Lisardo Boscá (CNIC) for helpful discussions, Eva Cano (CNIC), José Sánchez-Prieto and Magdalena Torres (Complutense University, Madrid, Spain) for advice on protein kinase experiments and their gift of the VASP antibody, Jesús Mateo (CNIC) for Tet-iNOS 293 cells used in this study, and Simon Bartlett (CNIC) for careful reading of this paper. This work was supported by the Spanish Ministry of Science grant BFI2003-03493 and by the Instituto de Salud Carlos III grant PI060701 awarded to S.C. Predoctoral fellowships from the Spanish Ministry of Science were received by E.A. and F.R.-J. (FPI) S.C. is funded by the ‘Ramón y Cajal’ programme.
References
- 1.Brookes P. S. Mitochondrial nitric oxide synthase. Mitochondrion. 2004;3:187–204. doi: 10.1016/j.mito.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol. Rev. 1987;39:163–196. [PubMed] [Google Scholar]
- 3.Ignarro L. J. Signal transduction mechanisms involving nitric oxide. Biochem. Pharmacol. 1991;41:485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- 4.Russwurm M., Koesling D. Guanylyl cyclase: NO hits its target. Biochem. Soc. Symp. 2004;71:51–63. doi: 10.1042/bss0710051. [DOI] [PubMed] [Google Scholar]
- 5.Russwurm M., Koesling D. NO activation of guanylyl cyclase. EMBO J. 2004;23:4443–4450. doi: 10.1038/sj.emboj.7600422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 7.Brown G. C., Cooper C. E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 8.Cleeter M. W., Cooper J. M., Darley-Usmar V. M., Moncada S., Schapira A. H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 9.Boveris A., Costa L. E., Poderoso J. J., Carreras M. C., Cadenas E. Regulation of mitochondrial respiration by oxygen and nitric oxide. Ann. N.Y. Acad. Sci. 2000;899:121–135. doi: 10.1111/j.1749-6632.2000.tb06181.x. [DOI] [PubMed] [Google Scholar]
- 10.Brookes P. S., Kraus D. W., Shiva S., Doeller J. E., Barone M. C., Patel R. P., Lancaster J. R., Jr, Darley-Usmar V. Control of mitochondrial respiration by NO, effects of low oxygen and respiration state. J. Biol. Chem. 2003;278:31603–31609. doi: 10.1074/jbc.M211784200. [DOI] [PubMed] [Google Scholar]
- 11.Brunori M., Forte E., Arese M., Mastronicola D., Giuffre A., Sarti P. Nitric oxide and the respiratory enzyme. Biochim. Biophys. Acta. 2006;1757:1144–1154. doi: 10.1016/j.bbabio.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Borutaite V., Brown G. C. Mitochondria rapidly reduce nitric oxide, and nitric oxide reversibly reduces mitochondrial respiration. Biochem. J. 1996;315:295–299. doi: 10.1042/bj3150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koivisto A., Matthias A., Bronnikov G., Nedergaard J. Kinetics of the inhibition of mitochondrial respiration by NO. FEBS Lett. 1997;417:75–80. doi: 10.1016/s0014-5793(97)01258-1. [DOI] [PubMed] [Google Scholar]
- 14.Clementi E., Brown G. C., Foxwell N., Moncada S. On the mechanism by which vascular endothelial cells regulate their oxygen consumption. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1559–1562. doi: 10.1073/pnas.96.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown G. C., Bolanos J. P., Heales S. J. R., Clark J. B. Nitric oxide produced by activated astrocytes rapidly and reversibly inhibits cellular respiration. Neurosci. Lett. 1995;193:201–204. doi: 10.1016/0304-3940(95)11703-y. [DOI] [PubMed] [Google Scholar]
- 16.Cooper C. E., Davies N. A., Psychoulis M., Canevari L., Bates T. E., Dobbie M. S., Casley C. S., Sharpe M. A. Nitric oxide and peroxynitrite cause irreversible increases in the Km for oxygen of mitochondrial cytochrome oxidase: in vitro and in vivo studies. Biochim. Biophys. Acta. 2003;1607:27–34. doi: 10.1016/j.bbabio.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Moncada S., Erusalimsky J. D. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 18.Stone J. R., Marletta M. A. Spectral and kinetic studies on the activation of soluble guanylate cyclase by nitric oxide. Biochemistry. 1996;35:1093–1099. doi: 10.1021/bi9519718. [DOI] [PubMed] [Google Scholar]
- 19.Russwurm M., Behrends S., Harteneck C., Koesling D. Functional properties of a naturally occurring isoform of soluble guanylyl cyclase. Biochem. J. 1998;335:125–130. doi: 10.1042/bj3350125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Artz J. D., Toader V., Zavorin S. I., Bennett B. M., Thatcher G. R. In vitro activation of soluble guanylyl cyclase and nitric oxide release: a comparison of NO donors and NO mimetics. Biochemistry. 2001;40:9256–9264. doi: 10.1021/bi002885x. [DOI] [PubMed] [Google Scholar]
- 21.Bellamy T. C., Garthwaite J. Sub-second kinetics of the nitric oxide receptor, soluble guanylyl cyclase, in intact cerebellar cells. J. Biol. Chem. 2001;276:4287–4292. doi: 10.1074/jbc.M006677200. [DOI] [PubMed] [Google Scholar]
- 22.Bellamy T. C., Wood J., Goodwin D. A., Garthwaite J. Rapid desensitization of the nitric oxide receptor, soluble guanylyl cyclase, underlies diversity of cellular cGMP responses. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2928–2933. doi: 10.1073/pnas.97.6.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffiths C., Garthwaite J. The shaping of nitric oxide signals by a cellular sink. J. Physiol. 2001;536:855–862. doi: 10.1111/j.1469-7793.2001.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellamy T. C., Griffiths C., Garthwaite J. Differential sensitivity of guanylyl cyclase and mitochondrial respiration to nitric oxide measured using clamped concentrations. J. Biol. Chem. 2002;277:31801–31807. doi: 10.1074/jbc.M205936200. [DOI] [PubMed] [Google Scholar]
- 25.Mateo J., García-Lecea M., Cadenas S., Hernández C., Moncada S. Regulation of hypoxia inducible factor-1α by nitric oxide through mitochondria-dependent and -independent pathways. Biochem. J. 2003;376:537–544. doi: 10.1042/BJ20031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J., Fritsch E. F., Maniatis T. 2nd edn. Plainview, NY: Cold Spring Harbor Laboratory Press; 1989. Molecular Cloning: a Laboratory Manual. [Google Scholar]
- 27.Borutaite V., Budriunaite A., Brown G. C. Reversal of nitric oxide-, peroxynitrite- and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols. Biochim. Biophys. Acta. 2000;1459:405–412. doi: 10.1016/s0005-2728(00)00178-x. [DOI] [PubMed] [Google Scholar]
- 28.Brown G. C. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett. 1995;369:136–139. doi: 10.1016/0014-5793(95)00763-y. [DOI] [PubMed] [Google Scholar]
- 29.Xia Y., Roman L. J., Masters B. S., Zweier J. L. Inducible nitric-oxide synthase generates superoxide from the reductase domain. J. Biol. Chem. 1998;273:22635–22639. doi: 10.1074/jbc.273.35.22635. [DOI] [PubMed] [Google Scholar]
- 30.Weaver J., Porasuphatana S., Tsai P., Pou S., Roman L. J., Rosen G. M. A comparative study of neuronal and inducible nitric oxide synthases: generation of nitric oxide, superoxide, and hydrogen peroxide. Biochim. Biophys. Acta. 2005;1726:302–308. doi: 10.1016/j.bbagen.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Schlossmann J., Hofmann F. cGMP-dependent kinases in drug discovery. Drug Discov. Today. 2005;10:627–634. doi: 10.1016/S1359-6446(05)03406-9. [DOI] [PubMed] [Google Scholar]
- 32.Mullershausen F., Russwurm M., Thompson W. J., Liu L., Koesling D., Friebe A. Rapid nitric oxide-induced desensitization of the cGMP response is caused by increased activity of phosphodiesterase type 5 paralleled by phosphorylation of the enzyme. J. Cell Biol. 2001;155:271–278. doi: 10.1083/jcb.200107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wykes V., Bellamy T. C., Garthwaite J. Kinetics of nitric oxide–cyclic GMP signalling in CNS cells and its possible regulation by cyclic GMP. J. Neurochem. 2002;83:37–47. doi: 10.1046/j.1471-4159.2002.01106.x. [DOI] [PubMed] [Google Scholar]
- 34.Liao J. K., Zulueta J. J., Yu F. S., Peng H. B., Cote C. G., Hassoun P. M. Regulation of bovine endothelial constitutive nitric oxide synthase by oxygen. J. Clin. Invest. 1995;96:2661–2666. doi: 10.1172/JCI118332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McQuillan L. P., Leung G. K., Marsden P. A., Kostyk S. K., Kourembanas S. Hypoxia inhibits expression of eNOS via transcriptional and posttranscriptional mechanisms. Am. J. Physiol. 1994;267:H1921–H1927. doi: 10.1152/ajpheart.1994.267.5.H1921. [DOI] [PubMed] [Google Scholar]
- 36.Xue C., Rengasamy A., Le Cras T. D., Koberna P. A., Dailey G. C., Johns R. A. Distribution of NOS in normoxic vs. hypoxic rat lung: upregulation of NOS by chronic hypoxia. Am. J. Physiol. 1994;267:L667–L678. doi: 10.1152/ajplung.1994.267.6.L667. [DOI] [PubMed] [Google Scholar]
- 37.Le Cras T. D., Xue C., Rengasamy A., Johns R. A. Chronic hypoxia upregulates endothelial and inducible NO synthase gene and protein expression in rat lung. Am. J. Physiol. 1996;270:L164–L170. doi: 10.1152/ajplung.1996.270.1.L164. [DOI] [PubMed] [Google Scholar]
- 38.Xue C., Johns R. A. Upregulation of nitric oxide synthase correlates temporally with onset of pulmonary vascular remodelling in the hypoxic rat. Hypertension. 1996;28:743–753. doi: 10.1161/01.hyp.28.5.743. [DOI] [PubMed] [Google Scholar]
- 39.Crawley D. E., Zhao L., Giembycz M. A., Liu S., Barnes P. J., Winter R. J., Evans T. W. Chronic hypoxia impairs soluble guanylyl cyclase-mediated pulmonary arterial relaxation in the rat. Am. J. Physiol. 1992;263:L325–L332. doi: 10.1152/ajplung.1992.263.3.L325. [DOI] [PubMed] [Google Scholar]
- 40.Hassoun P. M., Filippov G., Fogel M., Donaldson C., Kayyali U. S., Shimoda L. A., Bloch K. D. Hypoxia decreases expression of soluble guanylate cyclase in cultured rat pulmonary artery smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2004;30:908–913. doi: 10.1165/rcmb.2003-0287OC. [DOI] [PubMed] [Google Scholar]
- 41.Li D., Zhou N., Johns R. A. Soluble guanylate cyclase gene expression and localization in rat lung after exposure to hypoxia. Am. J. Physiol. 1999;277:L841–L847. doi: 10.1152/ajplung.1999.277.4.L841. [DOI] [PubMed] [Google Scholar]
- 42.Santolini J., Meade A. L., Stuehr D. J. Differences in three kinetic parameters underpin the unique catalytic profiles of nitric-oxide synthases I, II and III. J. Biol. Chem. 2001;276:48887–48898. doi: 10.1074/jbc.M108666200. [DOI] [PubMed] [Google Scholar]
- 43.Lee J., Ryu H., Ferrante R. J., Morris S. M., Jr, Ratan R. R. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y., Brandish P. E., DiValentin M., Schelvis J. P. M., Babcock G. T., Marletta M. A. Inhibition of soluble guanylate cyclase by ODQ. Biochemistry. 2000;39:10848–10854. doi: 10.1021/bi9929296. [DOI] [PubMed] [Google Scholar]
- 45.Stasch J.-P., Becker E. M., Alonso-Alija C., Apeler H., Dembowsky K., Feurer A., Gerzer R., Minuth T., Perzborn E., Pleiss U., et al. NO-independent regulatory site on soluble guanylate cyclase. Nature. 2001;410:212–215. doi: 10.1038/35065611. [DOI] [PubMed] [Google Scholar]
- 46.Gnaiger E., Kuznetsov A. V. Mitochondrial respiration at low levels of oxygen and cytochrome c. Biochem. Soc. Trans. 2002;30:252–258. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 47.Gnaiger E., Lassnig B., Kuznetsov A., Rieger G., Margreiter R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J. Exp. Biol. 1998;201:1129–1139. doi: 10.1242/jeb.201.8.1129. [DOI] [PubMed] [Google Scholar]
- 48.Rossignol R., Faustin B., Rocher C., Malgat M., Mazat J. P., Letellier T. Mitochondrial threshold effects. Biochem. J. 2003;370:751–762. doi: 10.1042/BJ20021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gladwin M. T., Raat N. J., Shiva S., Dezfulian C., Hogg N., Kim-Shapiro D. B., Patel R. P. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signalling, cytoprotection, and vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 50.Gnaiger E. Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir. Physiol. 2001;128:277–297. doi: 10.1016/s0034-5687(01)00307-3. [DOI] [PubMed] [Google Scholar]
- 51.Brand M. D., Murphy M. P. Control of electron flux through the respiratory chain in mitochondria and cells. Biol. Rev. 1987;62:141–193. doi: 10.1111/j.1469-185x.1987.tb01265.x. [DOI] [PubMed] [Google Scholar]
- 52.Brown G. C. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem. J. 1992;284:1–13. doi: 10.1042/bj2840001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palacios-Callender M., Quintero M., Hollis V. S., Springett R. J., Moncada S. Endogenous NO regulates superoxide production at low oxygen concentrations by modifying the redox state of cytochrome c oxidase. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7630–7635. doi: 10.1073/pnas.0401723101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deguchi A., Soh J. W., Li H., Pamukcu R., Thompson W. J., Weinstein I. B. Vasodilator-stimulated phosphoprotein (VASP) phosphorylation provides a biomarker for the action of exisulind and related agents that activate protein kinase G. Mol. Cancer Ther. 2002;1:803–809. [PubMed] [Google Scholar]
- 55.Andre M., Latado H., Felley-Bosco E. Inducible nitric oxide synthase-dependent stimulation of PKGI and phosphorylation of VASP in human embryonic kidney cells. Biochem. Pharmacol. 2005;69:595–602. doi: 10.1016/j.bcp.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 56.Kemp B. E., Stapleton D., Campbell D. J., Chen Z. P., Murthy S., Walter M., Gupta A., Adams J. J., Katsis F., van Denderen B., et al. AMP-activated protein kinase, super metabolic regulator. Biochem. Soc. Trans. 2003;31:162–168. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- 57.Hawley S. A., Davison M., Woods A., Davies S. P., Beri R. K., Carling D., Hardie D. G. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]