Abstract
The in vivo response of the immune system after HIV infection in regard to cytokine production and C-C chemokine synthesis is not well known. Here we have analysed cytokine and chemokine mRNA production in lymph nodes with follicular hyperplasia (FHLN) of HIV-infected patients by in situ hybridization using anti-sense mRNA probes. The synthesis of mRNAs for interferon-gamma (IFN-γ), IL-12p35, IL-12p40, IL-4, and for the C-C chemokines RANTES, MIP-1α, and MIP-1β was compared with that of lymph nodes from non-infected individuals to define HIV-specific events. Only few cells expressing IFN-γ, RANTES, MIP-1α, and MIP-1β mRNAs were detectable in the T-dependent area of lymph nodes from HIV-negatives. In contrast, in FHLN from HIV+ patients a high number of IFN-γ, RANTES, MIP-1α, and MIP-1β mRNA-containing cells were detectable. Remarkably, only single individual IL-12p35 mRNA-producing cells were present in the T-dependent area from both HIV+ and HIV− lymph nodes. Furthermore, the low number of IL-12p40 mRNA-expressing cells did not differ between HIV+ and HIV− lymph nodes. This indicates that IFN-γ is expressed independently of IL-12, possibly by a direct T cell-mediated reaction. IL-4 mRNA-producing cells were hardly detectable in infected and control lymph nodes. The same findings were made in a limited number of samples from patients with advanced disease. Thus, these results demonstrate that a high IFN-γ production is accompanied by a strong expression of MIP-1α, MIP-1β, and RANTES in the lymph node after HIV infection. This favours the idea that a Th1-type immune response correlates with a preferential production of C-C chemokines in FHLN of HIV+ patients.
Keywords: HIV-1, lymph node, cytokines, C-C chemokines, in situ hybridization
INTRODUCTION
The most obvious and dramatic immunologic change that occurs during progression of an HIV infection to AIDS is the severe depletion of CD4+ T cells in the blood and in lymphoid tissue. However, long before a decline in the number of circulating CD4+ T cells is obvious a loss of the T helper (Th) cell function is observed in HIV+ individuals [1], indicating that factors other than CD4 depletion contribute to T cell dysfunction. As a popular hypothesis it has been put forward that a switch from the Th1 to the Th2 cytokine phenotype is a critical step in the progression of HIV disease. After in vitro stimulation of unfractionated peripheral blood mononuclear cells (PBMC) from HIV-infected individuals with phytohaemagglutinin (PHA) or recall antigen, production of IL-4 and IL-10 increased with disease progression [2,3]. However, controversial results have been reported [4] that demonstrate that IL-4 expression was barely detectable or undetectable regardless of the stage of disease in unfractionated and sorted cell populations isolated from peripheral blood and lymph nodes. Also, CD8+ cells stably expressed large amounts of interferon-gamma (IFN-γ) and IL-10 throughout the course of infection and CD4+ T cells from HIV+ individuals stimulated in vitro showed a similar cytokine expression at different stages of the disease. Maggi et al. [5] underlined these data by generating T cell clones from HIV+ patients. A preferential reduction in clones producing IL-4 and IL-5 in advanced phases of infection was obvious. Moreover, a large proportion of these CD4+ T cell clones produced both Th1 and Th2 cytokines. Thus, HIV does not induce a definite Th1 to Th2 switch, but favours a shift to the Th0 phenotype in response to recall antigen.
Despite this controversial discussion of the Th1/Th2 concept in AIDS, it is well documented that cytokines directly affect HIV replication. While the Th1 cytokine IFN-γ enhances cellular immune responses against HIV, the Th2 cytokines IL-4 and IL-10 suppress HIV replication [6]. Recent studies demonstrate that chemokines, a superfamily of small structurally related proteins, exhibiting proinflammatory properties and involved in the recruitment and activation of leucocytes to inflammatory lesions [7], can also regulate HIV replication. The C-C chemokines RANTES (regulated on activation normal T cell expressed and secreted), MIP-1α, and MIP-1β, produced by both primary and in vitro immortalized CD8+ T cells, are synergistically effective in the inhibition of the replication of monocyte/macrophage-tropic HIV-1 strains [8].
Most of these studies in regard to cytokine expression in HIV patients were performed with PBMC, isolated lymph node cells or T cell clones stimulated in vitro. Lymph nodes of HIV-infected individuals are the major site of HIV replication [9–15], especially during the clinically latent phase of the disease. Therefore, we analysed by in situ hybridization the number, phenotype and localization in the lymph node of cells producing the cytokines IFN-γ, IL-12p35, IL-12p40 and IL-4, and the chemokines MIP-1α, MIP-1β, and RANTES. The synthesis of these cytokines and chemokines was compared between lymph nodes with follicular hyperplasia (FHLN) from HIV-infected and lymph nodes from non-infected individuals. Our results indicate: (i) that HIV preferentially induces a strong IL-12-independent IFN-γ immune response, and (ii) that the high IFN-γ mRNA expression correlates with a high C-C chemokine production in HIV-replicating lymph nodes.
PATIENTS AND METHODS
Patients
Eight cases of follicular hyperplasia associated with HIV-1 infection and two cases with late stage HIV infection were retrieved from the files of the Department of Pathology. The most important clinical data are summarized in Table 1. None of the patients had opportunistic infections. For control, three lymph nodes from HIV− individuals were investigated. Lymph nodes from all individuals were removed for diagnostic purposes. Five micrometre thick cryostat sections were prepared and used for in situ hybridization and immunohistochemistry.
Table 1.
Characteristics and clinical details of HIV-1-infected patients
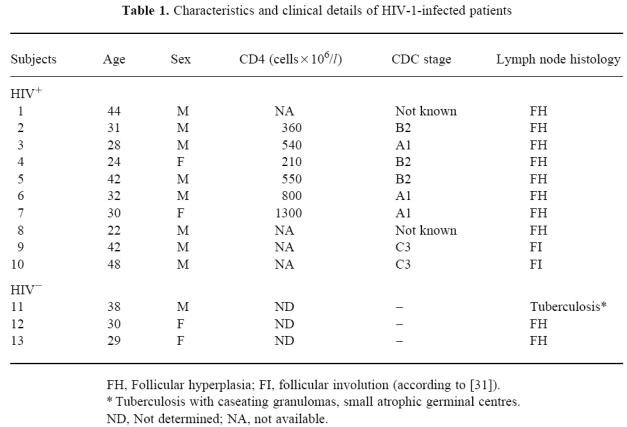
FH, Follicular hyperplasia; FI, follicular involution (according to [31]).
DNA probes and in vitro transcription
cRNA probes were used for detection of cytokine mRNAs. The sizes of the sense and anti-sense probes were for IFN-γ 437 bp, for MIP-1α 194 bp, for MIP-1β 185 bp, for RANTES 161 bp, for IL-12p35 590 bp, for IL-12p40 673 bp, and for IL-4 386 bp. Subcloning of specific cDNA fragments in the Bluescript KS plasmid (Stratagene, Heidelberg, Germany) with T3/T7 initiation sites was done by standard protocols. In vitro transcription of sense and antisense probes was performed essentially as described recently [16].
In situ hybridization
In situ hybridization was performed exactly as described previously [16] on 5 μm cryostat sections. Coated tissues were developed and fixed after 16 days and counterstained with haemalaun. As negative controls, sections of each lymph node were hybridized with 35S-labelled sense probes. To control the reactivity of the respective cRNA probes, they were used on p35- and p40-transfected chinese hamster ovary (CHO) cells and on PHA-stimulated peripheral blood lymphocytes (PBL). Two sections from the same biopsy were hybridized with every antisense and sense probe in three independent experiments. Each lymph node specimen was analysed for the production of all cytokines, but only representative results are shown in the figures. In each probe and each patient, lymph node cytokine/chemokine-producing cells were counted. Results are expressed as the mean number (± s.d.) of lymphokine/chemokine-producing cells per cm2 of two sections analysed.
Immunohistological staining
Cryostat sections (5 μm) were subjected to immunohistochemistry using an alkaline phosphatase anti-alkaline phosphatase method, as described [17]. CD4+ cells were detected by a mixture of antibodies OKT4 (OrthoDiagnostic System, Neckargemünd, Germany), CD4-1F6 (Medac, Hamburg, Germany) and Leu-3a (Becton Dickinson, Heidelberg, Germany); CD8+ cells were detected with Leu-2a (Becton Dickinson) and CD8/144B (Dako, Hamburg, Germany) and macrophages by MoAb CD68/KP1 (Dako). To determine the specific cell type(s) producing IFN-γ and MIP-1α mRNAs, in situ hybridization studies were performed after immunohistochemical staining of the sections.
RESULTS
Detection and distribution of IFN-γ and IL-4 mRNA-expressing cells with HIV-infected and control lymph nodes
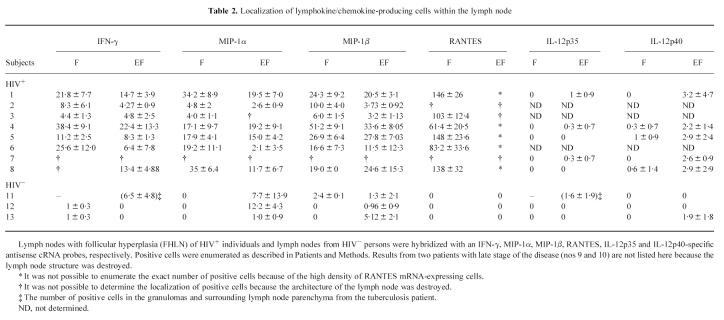
An IFN-γ-specific antisense riboprobe was used to detect IFN-γ mRNA-expressing cells in sections from the eight HIV+ and the HIV− lymph nodes. Only a few IFN-γ mRNA-producing cells were seen in two HIV− lymph nodes (Fig. 1A). These cells were predominantly located in the follicle (Table 2). In the lymph node from the tuberculosis patient (patient 11) the number of IFN-γ-positive cells was increased, demonstrating that in this bacterial infection IFN-γ mRNA was up-regulated in the granulomas (Fig. 1B). Labelled cells were also found in the surrounding lymph node parenchyma. Interestingly a high number of IFN-γ mRNA-positive cells was detected in all HIV+ lymph nodes (Fig 1C). Most cells expressing IFN-γ were located in the germinal centres or within the inner part of the mantle zone and to a lower level in the T-dependent zone (Table 2), characterized by the presence of high endothelial venules and interdigitating cells.
Fig. 1.
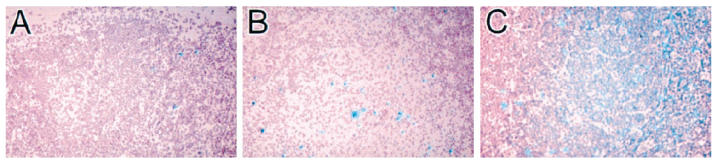
IFN-γ mRNA production in HIV− and HIV+ lymph nodes. Lymph nodes with follicular hyperplasia (FHLN) of HIV+ individuals and lymph nodes from HIV− persons were hybridized with an IFN-γ-specific antisense cRNA probe. (A) Lymph node section from a HIV− individual. (B) Lymph node section from a Mycobacterium tuberculosis-infected but HIV− patient. (C) Lymph node section from a HIV+ person (Mag. × 50).
Table 2.
Localization of lymphokine/chemokine-producing cells within the lymph node
Lymph nodes with follicular hyperplasia (FHLN) of HIV+ individuals and lymph nodes from HIV− persons were hybridized with an IFN-γ, MIP-1α, MIP-1β, RANTES, IL-12p35 and IL-12p40-specific antisense cRNA probes, respectively. Positive cells were enumerated as described in Patients and Methods. Results from two patients with late stage of the disease (nos 9 and 10) are not listed here because the lymph node structure was destroyed.
To identify the IFN-γ-producing cell type in the tissue of HIV patients, we used a combined immunohistochemistry and in situ hybridization method. As shown in Fig. 2A,B, both CD4+ and CD8+ lymphocytes produced IFN-γ mRNA. Thus, our results support and extend data from Emilie et al. [14], who demonstrated IFN-γ production by CD8+ cells in HIV+ lymph nodes.
Fig. 2.

Identification of the cell types producing IFN-γ and MIP-1α mRNA. Lymph node sections were subsequently labelled with anti-CD4 (A, C), anti-CD8 (B, D), and anti-CD68 (E), respectively, and hybridized with an IFN-γ (A, B) or MIP-1α (C, D, E)-specific riboprobe. Double-positive cells were marked with an arrow. (Mag. A × 168, B–E × 100.)
It is of note that when the production of IL-4 in HIV+ lymph nodes was analysed, positive cells were hardly detectable. The same held true for HIV− lymph nodes (data not shown).
Detection and distribution of IL-12p35 and IL-12p40 mRNA-expressing cells in HIV-infected and control lymph nodes
Since IL-12 plays an important role in the production of IFN-γ by natural killer (NK) cells and T cells, we investigated the in situ expression of IL-12. Few single cells were positive for IL-12p35 signals in lymph nodes of both HIV− and HIV+ individuals (Table 2). In all tissues examined, rarely IL-12p40 mRNA-positive cells were seen. The predominant localization of IL-12p40 mRNA was in the T-dependent area. However, in some HIV+ tissues a few cells staining with the labelled riboprobe were located in the germinal centres or within the mantle zone. Thus, it seems that the strong IFN-γ response induced by HIV is mediated in an IL-12-independent way.
Detection and distribution of MIP-1α, MIP-1β, and RANTES mRNA-expressing cells in HIV-infected and control lymph nodes
Lymph node sections were hybridized with MIP-1α-, MIP-1β- and RANTES-specific probes. In all lymph nodes from HIV− individuals, only a few individual MIP-1α-positive cells were detected in T-dependent and interfollicular areas, whereas the germinal centres showed no labelling (Fig. 3A and Table 2). However, MIP-1α mRNA was strongly expressed and predominantly located in germinal centres (Fig. 3B) and T-dependent areas (Fig. 3C) in HIV+ FHLN. By using a double-labelling technique, two cell types expressing MIP-1α mRNA were detected. In the germinal centres MIP-1α-positive cells were identified as CD68+ macrophages (Fig. 2E). In addition, large CD4+ cells with broad cytoplasm also expressed MIP-1α (Fig. 2C). The cell morphology suggested that these cells were macrophages as well. Also, CD8+ lymphocytes expressed MIP-1α mRNA (Fig. 2D). They were located in the paracortex.
Fig. 3.
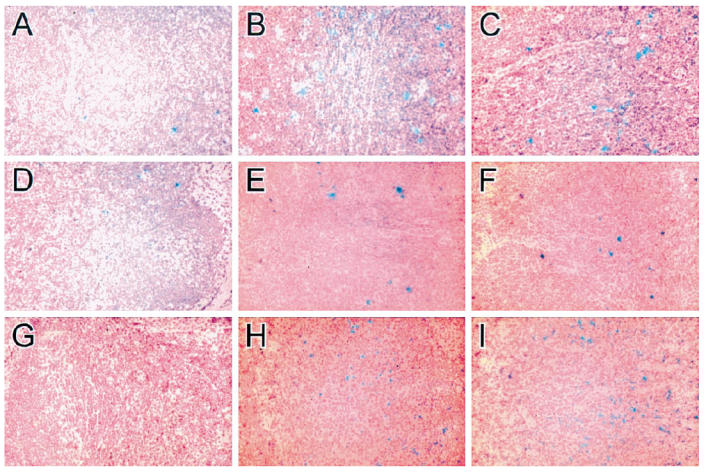
MIP-1α, MIP-1β, and RANTES mRNA production in HIV− and HIV+ lymph nodes. Lymph nodes with follicular hyperplasia (FHLN) from HIV+ individuals and lymph nodes from HIV− persons were hybridized with MIP-1α (A–C)-, MIP-1β (D–F)-, and RANTES (G–I)-specific antisense cRNA probes. (A,D,G) Lymph node sections from an HIV− individual. (B,E,H) Germinal centre in the lymph node from an HIV+ patient. (C,F,I) T-dependent area in the lymph node from an HIV+ person. (Mag. × 50.)
MIP-1β mRNA was strongly expressed in the germinal centres (Fig. 3E) and in the T-dependent zones (Fig. 3F) of the HIV lymph nodes, in comparison with an infrequent expression in the control lymph nodes (Fig. 3D and Table 2). The contrast between the expression of RANTES mRNA in lymph nodes of HIV-infected individuals and HIV− lymph nodes was remarkable. No positive cells were seen in the controls (Fig. 3G). In all HIV-1+ lymph nodes examined, RANTES-positive cells were localized in the germinal centres (Fig. 3H) and, in extremely high amounts, also in the T-dependent areas (Fig. 3I and Table 2). No labelling was found on endothelial cells of the high endothelial venules or in lymphocytes present in the lumen of these venules. These data are in contrast to findings by Tedla et al. [18], who reported a high expression of RANTES even at this location in control lymph nodes.
Thus, in FHLN of HIV+ individuals a strong expression of MIP-1α, MIP-1β and RANTES was evident, indicating a close correlation between a preferential Th1 cytokine expression and C-C chemokine synthesis in HIV-infected patients.
Analysis of lymph nodes from AIDS patients
The lymphoid tissue studied so far was at a relatively early/mild stage of disease progression. We have in addition studied two lymph nodes from two HIV patients (nos 9 and 10) in a late stage of disease. The lymph node structure was largely destroyed in this tissue. Here essentially the same results were obtained: many cells positive for IFN-γ, MIP-1β and MIP-1α mRNA were detectable in the absence of IL-12 mRNA, and no positive IL-4 staining was evident (data not shown).
DISCUSSION
In this study we have investigated the number, distribution and phenotype of cells expressing mRNA of the cytokines IFN-γ, IL-12p35, IL-12p40, and IL-4 and of the C-C chemokines MIP-1α, MIP-1β and RANTES in HIV-infected and non-infected lymph nodes, the most relevant compartment for virus multiplication and antiviral immune responses.
A high number of IFN-γ mRNA-producing cells was present in HIV+ lymph nodes, while in HIV-negatives only a few cells showed positive hybridization signals. The IFN-γ-positive cells were located in the extrafollicular parenchyma and in the germinal centres. Both CD4+ and CD8+ lymphocytes contributed to the strong IFN-γ mRNA expression. A slight increase of positive cells was visible in lymph nodes from the HIV− individual infected by Mycobacterium tuberculosis, demonstrating that IFN-γ production was enhanced by this infectious agent, but the stimulus was less strong than that given by HIV. These data are in agreement with an earlier study by Emilie et al. [14] and have particular relevance, since they clearly show that in HIV infection T cells are present that spontaneously produce the Th1 cytokine IFN-γin situ. No IL-4 mRNA-producing cells were detectable in lymph nodes either of infected or non-infected patients.
It should be emphasized that most of the lymph nodes under study were from patients at a relatively early/mild stage of immunological progression. However, the two lymph nodes from HIV patients in a late stage of the disease studied showed essentially the same predominance of IFN-γ and the chemokines MIP-1α and MIP-1β mRNA production, with no IL-4-producing cells. Although these data are generated from only two patients, they are in contrast to previously published data, generated by the use of in vitro stimulated peripheral blood cells or lymph node cells, and demonstrating that HIV progression is associated with a loss of type 1 responses and an increase in type 2 responses [2,3].
The decisive cytokine, stimulating Th1 cell responses and increasing induction of IFN-γ, is IL-12, a heterodimeric protein of 70 kD formed by two covalently linked chains of 35 kD (p35) and 40 kD (p40). While p35 is constitutively expressed, p40 is strongly affected by stimulation with bacteria, bacterial products or intracellular parasites [19]. Interestingly, PBMC from HIV-positives not only show decreased p35 and p40 mRNA expression, but also produce less IL-12 than cells from HIV− donors [20–22] after in vitro stimulation with Staphylococcus aureus. Since alveolar macrophages from HIV+ patients constitutively produce higher levels of IL-12 than do those from healthy donors [23], it is believed that phagocytic cells from HIV-infected patients are in a state of activation in vivo characterized by constitutive expression of low levels of IL-12. Our results show that IL-12 was only produced at very low levels in the lymph nodes of HIV− persons, and that neither IL-12p35 mRNA nor IL-12p40 mRNA were increased in HIV+ individuals. Thus, IL-12 does not seem to play a central role in inducing IFN-γ, the major cytokine expressed in the FHLN. Therefore, this IFN-γ is probably a product of an antigen-specific reaction of T lymphocytes.
A strong induction of the chemokine genes for MIP-1α, MIP-1β and RANTES was observed in lymph nodes from the HIV+ individuals. Interestingly, the strong expression of C-C chemokine messages and the high production of IFN-γ mRNA within the same lymph nodes indicate a direct correlation between IFN-γ/Th1 immune responses and C-C chemokine expression. It is of note that IFN-γ, MIP-1α and MIP-1β were similarly localized within the lymph node compartments. Thus, these results confirm and extend an earlier study describing that C-C chemokines are preferentially produced by Th1 cells in vitro [24].
Since MIP-1α and MIP-1β are up-regulated in monocytes in vitro and in vivo as a direct consequence of HIV infection [25,26], the strong chemokine gene expression may be a direct consequence of the viral replication in the lymph node. The influence of the chemokines on the course of infection might be mediated by affecting viral replication and by recruiting circulating immune cells in lymphoid organs. It is well established that MIP-1α, MIP-1β and RANTES possess suppressive activities resulting in the down-regulation of HIV replication of the monocyte/macrophage-tropic HIV-1 strains [8]. Since these C-C chemokines were produced at high amounts in the FHLN early and late in infection, it is obvious that disease can progress despite high chemokine production. This assumption was underlined by recently published data demonstrating that C-C chemokines are not present in higher concentrations in CD8+ cell culture supernatants from HIV-infected long term survivors compared with those from individuals in whom the disease is progressing [27].
During HIV infection increased numbers of macrophages and lymphocytes traffic into the lymph nodes. Based on their leucocyte chemoattractant and activating properties the chemokines MIP-1α, MIP-1β and RANTES may contribute to this cell accumulation. As shown here, CD68+ and CD4+ cells exhibiting a macrophage-like morphology within the germinal centre, and CD8+ lymphocytes in the T-dependent zone produced MIP-1α. Interestingly, MIP-1α is the chemoattractant for CD8+ T cells and also CD4+ T cells [28,29]. As reported earlier by ourselves [30], in lymph nodes with explosive follicular hyperplasia an accumulation of CD8+ lymphocytes in the lymph node occurred. Thus, the chemokines, by recruiting the cells to the site of virus replication, may be involved in creating an inflammatory cell-rich milieu in the lymph node and thus play a pivotal role in the pathophysiology of HIV infection.
Acknowledgments
This work was supported by the Bundesministerium für Forschung und Technologie, Germany. We thank G. Großschupf and A. Veit for expert technical assistance.
References
- 1.Clerici M, Stocks NI, Zajac RA, et al. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. J Clin Invest. 1989;84:1892–9. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clerici M, Hakim FT, Venzon DJ, et al. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Invest. 1993;91:759–65. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clerici M, Wynn TA, Berzofsky JA, et al. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest. 1994;93:768–75. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graziosi C, Pantaleo G, Gantt KR, et al. Lack of evidence for the dichotomy of TH1 and TH2 predominance in HIV-infected individuals. Science. 1994;265:248–52. doi: 10.1126/science.8023143. [DOI] [PubMed] [Google Scholar]
- 5.Maggi E, Mazzetti M, Ravina A, et al. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science. 1994;265:244–8. doi: 10.1126/science.8023142. [DOI] [PubMed] [Google Scholar]
- 6.Mackewicz CE, Ortega H, Levy JA. Effect of cytokines on HIV replication in CD4+ lymphocytes: lack of identity with the CD8+ cell antiviral factor. Cell Immunol. 1994;153:329–43. doi: 10.1006/cimm.1994.1032. [DOI] [PubMed] [Google Scholar]
- 7.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Ann Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 8.Cocchi F, DeVico AL, Garzino Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–5. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 9.Fox GH, Tenner-Racz K, Racz P, Firpo A, Pizzo PA, Fauci AS. Lymphoid germinal centers are reservoirs of human immunodeficiency virus type 1 RNA. J Infect Dis. 1991;164:1051–7. doi: 10.1093/infdis/164.6.1051. [DOI] [PubMed] [Google Scholar]
- 10.Pantaleo G, Graziosi C, Butini L, et al. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88:9838–42. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Embretson J, Zupancic M, Ribas JL, et al. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;62:359–62. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 12.Pantaleo G, Graziosi C, Demarest JF, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–8. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 13.Tenner-Racz K, Racz P, Schmidt H, et al. Immunohistochemical, electron microscopic and in situ hybridization evidence for the involvement of lymphatics in the spread of HIV-1. AIDS. 1988;2:299–309. doi: 10.1097/00002030-198808000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Emilie D, Peuchmaur M, Maillot MC, et al. Production of interleukins in human immunodeficiency virus-1-replicating lymph nodes. J Clin Invest. 1990;86:148–59. doi: 10.1172/JCI114678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tenner-Racz K, Bofill H, Schulz-Meyer A, et al. HTLV-II/LAV viral antigens in lymph nodes of homosexual men with persistent generalized lymphadenopathy and AIDS. Am J Pathol. 1986;123:9–15. [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer zum Büschenfelde C, Cramer S, Trumpfheller C, Fleischer B, Frosch S. Trypanosoma cruzi induces strong IL-12 and IL-18 gene expression in vivo: correlation with interferon-gamma (IFN-γ) production. Clin Exp Immunol. 1997;110:378–85. doi: 10.1046/j.1365-2249.1997.4471463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordell JL, Falini B, Erber WN, et al. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes) J Histochem Cytochem. 1984;32:219–29. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- 18.Tedla N, Palladinetti P, Kelly M, et al. Chemokines and T lymphocyte recruitment to lymph nodes in HIV infection. Am J Pathol. 1996;148:1367–73. [PMC free article] [PubMed] [Google Scholar]
- 19.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-γ) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 20.Gazzinelli RT, Bala S, Stevens R, et al. HIV infection suppresses type 1 lymphokine and IL-12 responses to Toxoplasma gondii but fails to inhibit the synthesis of other parasite-induced monokines. J Immunol. 1995;155:1565–74. [Google Scholar]
- 21.Chougnet C, Wynn TA, Clerici M, et al. Molecular analysis of decreased interleukin-12 production in persons infected with immunodeficiency virus. J Infect Dis. 1996;174:46–53. doi: 10.1093/infdis/174.1.46. [DOI] [PubMed] [Google Scholar]
- 22.Clerici M, Lucey DR, Berzofsky JA, et al. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science. 1993;262:1721–4. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- 23.Denis M, Ghadirian E. Dysregulation of interleukin 8, interleukin 10, and interleukin 12 release by alveolar macrophages from HIV type 1-infected subjects. AIDS Res Hum Retrovir. 1994;10:1619–27. doi: 10.1089/aid.1994.10.1619. [DOI] [PubMed] [Google Scholar]
- 24.Schrum S, Probst P, Fleischer B, Zipfel PF. Synthesis of the CC-chemokines MIP-1alpha, MIP-1beta, and RANTES is associated with a type 1 immune response. J Immunol. 1996;157:3598–604. [PubMed] [Google Scholar]
- 25.Schmidtmayerova H, Nottet HS, Nuovo G, et al. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci USA. 1996;93:700–4. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canque B, Rosenzwajg M, Gey A, Tartour E, Fridman WH, Gluckman JC. Macrophage inflammatory protein-1alpha is induced by human immunodeficiency virus infection of monocyte-derived macrophages. Blood. 1996;87:2011–9. [PubMed] [Google Scholar]
- 27.Mackewicz CE, Barker E, Levy JA. Role of beta-chemokines in suppressing HIV replication. Science. 1996;274:1393–5. doi: 10.1126/science.274.5291.1393. [DOI] [PubMed] [Google Scholar]
- 28.Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260:355–8. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 29.Schall TJ, Bacon K, Camp RD, Kaspari JW, Goeddel DV. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–6. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Racz P, Tenner-Racz K, van Vloten F, et al. Lymphatic tissue changes in AIDS and other retrovirus infections: tools and insights. Lymphology. 1990;23:85–91. [PubMed] [Google Scholar]
- 31.Knowles DM. Baltimore: Williams and Wilkins; 1992. Neoplastic hematopathology. [Google Scholar]