Abstract
Oral and more recently nasal tolerance have attracted attention as potential treatments of autoimmune disease. Arthritis induced by bovine type II collagen (CII) is a widely used animal model of rheumatoid arthritis, which is here used to investigate the efficacy of nasal treatment by a short peptide. The peptide spans residues 707–721 (designated p707), an epitope of mouse CII that is most strongly recognized after immunization of mice with this self-protein. The treatment was partially effective, but almost only when the peptide was administered in large doses over a prolonged period. Mice immunized with bovine CII respond mainly to other peptides, located in the CB11 fragment around amino acid residues 256–270. The tolerance effect therefore results from intramolecular suppression, between epitopes located in different parts of this large protein.
Keywords: bystander suppression, collagen-induced arthritis, self epitope
INTRODUCTION
Bystander suppression occurs when an immune response to one epitope suppresses the response to another, as a consequence of one of the two epitopes occurring in the vicinity of the other. This is a regulatory mechanism of importance in autoimmunity, where the suppressive response can be elicited by mucosal administration of antigen [1–3]. It is an attractive strategy for therapy, since although the disease-inducing antigen in organ-specific autoimmune disease is often unknown, other organ-specific antigens can be selected for mucosal administration.
The bystander suppressive effect is associated with production by the suppressive cells of the cytokines transforming growth factor-beta (TGF-β) and IL-4 [4,5], and also IL-10 [6,7], although there are additional mechanisms of oral tolerance which do not involve bystander suppression [8]. The mechanism is similar in principle to epitope-linked help between T cells [9], where both cytokine [10,11] and epicrine [12,13] mechanisms are known to operate. An important distinction can be made between intra- and intermolecular effects, according to whether the two epitopes are presented on the same or different proteins [9]. Bystander suppression of intermolecular type has been shown to protect against experimental allergic encephalomyelitis (EAE) [7]; it can also protect non-obese diabetic (NOD) mice against diabetes, since peptides from either insulin [14] or glutamic acid decarboxylase (GAD) [5] could both protect.
Nasal administration of peptide has proved a reliable means of suppressing autoimmune disease [7,15–17], and in the Metzler & Wraith studies is clearly superior to oral administration. Furthermore, aerosolization was more effective than oral administration of myelin basic protein in suppressing EAE [18]. Accordingly, nasal administration was chosen for use in the present study.
Collagen-induced arthritis (CIA) in mice is a typical organ-specific autoimmune disease induced with Freund's complete adjuvant (FCA), which is characterized by a preponderance of Th1-type cells in the progression phase [19], although IL-4 is essential for initial triggering [20]. T cell recognition of epitopes within the CB11 fragment of type II collagen (CII), particularly of p256–270, is crucial for the development of CIA induced with foreign CII [21].
Our recent study of T cell responses to autologous (mouse) CII identified a second major epitope, spanning residues 707–721 (p707) [22]. The present study had two purposes, to test (i) whether treatment with this short peptide is able to prevent or ameliorate the disease, and (ii) whether intramolecular bystander suppression occurs between the two epitopes.
MATERIALS AND METHODS
Peptide synthesis
The mouse CII peptide 707–721 (PPG ANG NPG PAG PPG) and rat CII peptide 256–270 (GEPG IAG FKG EQG PK) were synthesized using standard Fmoc chemistry (Applied Biosystems (Foster City, CA) Peptide Synthesizer; 432 A-Synergy) and purified by reversed phase high-performance liquid chromatography (HPLC). The structures were confirmed by mass spectroscopy.
Preparation of CII
Mouse CII was extracted from xiphisterna by pepsin digestion [23], and further purified by salt precipitation [24]. Bovine CII was prepared from nasal cartilage as described previously [24]. The collagens were dissolved in 0.1 m acetic acid.
Induction and scoring of arthritis
Arthritis was induced in male (DBA/1 × B10.Q) F1 mice, 8–12 weeks of age, by immunizing at the base of the tail with 100 μg bovine CII emulsified 1:1 in 100 μl Freund's incomplete adjuvant (FIA; Difco, Detroit, MI) containing 800 μg autoclaved Mycobacterium tuberculosis (gift from the Ministry of Agriculture, Fisheries and Food Laboratory, Weybridge, UK) [20,25]. The mice were boosted 3 weeks later with the same amount of collagen in PBS. Arthritis was scored starting 1 week later for a period of 8 weeks as follow: 1, erythema of toes; 2, erythema and swelling of paws; 3, swelling of ankles; 4, swelling and impaired mobility of whole leg. The arthritis score was determined by an observer unaware of the experimental design, and then averaged over the entire period of observation. The group means shown are for all the averaged individual scores; a non-parametric test was applied, to allow for inclusion of individuals who did not develop arthritis.
Induction of tolerance by intranasal application of peptides
Peptide or collagen dissolved in 50 μl PBS were instilled into the nostrils of mice under light ether anaesthesia, in groups of 7–12 animals. Control groups received 50 μl PBS alone. Doses and intervals are indicated in figure legends.
Neutrophil chemiluminescence
Chemiluminescence was determined on 100-μl samples of whole blood according to published methods [24].
RESULTS
Short-term treatment with p707
To investigate the effect of nasal administration of the mouse CII peptide p707–721 on the development of CIA, groups of 10–12 male (DBA/1 × B10.Q) F1 mice were treated with peptide 14 days before immunization with CII at doses and quantity of applications as indicated in the legend of Fig. 1. Groups of the same size were also treated with intact bovine CII (the disease-inducing agent), mouse CII or the immunodominant foreign CII epitope 256–270. Among the groups treated with a single dose of p707, one group that received 10 μg peptide showed strong suppression of CIA (group 4* in Fig. 1). The results of this experiment are shown in detail in Fig. 2. The effect, however, was not repeatable in other experiments, as shown in Fig. 1, thus leaving this exceptional result unaccounted for. Nor was suppression seen in other single-dose treatments at 30 μg or 1 μg of peptide. Some variation of mean arthritis scores was evident in the control groups treated with PBS alone, in the range 2.2–3.8, as has been observed in previous studies of our own and of others [6,16,20,21,25].
Fig. 1.
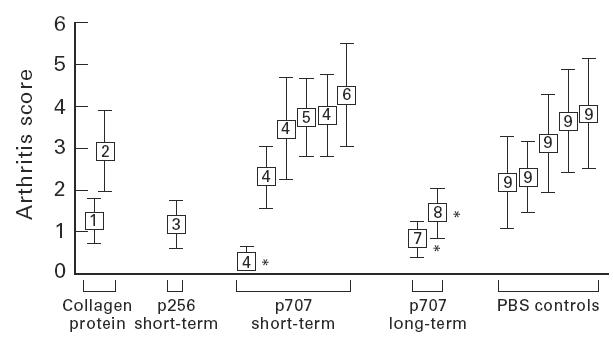
Effect of various forms of nasal administration of collagen type II (CII) peptides on the development of collagen-induced arthritis (CIA). Arthritis was induced in mice by injection with bovine CII as described in Materials and Methods. Dose of peptide and intervals of intranasal antigen applications of different groups in relation to time of the first disease-inducing injection were as follows: (i) 10 μg mouse CII, week −2, −1; (ii) 10 μg bovine CII, week −2, −1; (iii) 10 μg p256, week −2, −1; (iv) 10 μg p707, week −2, −1 or −1 only; (v) 1 μg p707, week −2, −1; (vi) 30 μg p707, week −2, −1; (vii) 10 μg p707, week −4, −3, −2, −1 and weeks +1, +2, +3, +4, +5, +6, +7, +8; (viii) 10 μg p707, week +1, +2, +3, +4, +5, +6, +7, +8; (ix) PBS only, to match treated groups. Mean arthritis scores of groups, measured over weeks 1–8 post second disease-inducing injection, are shown. *Groups shown in fuller detail in Fig. 2.
Fig. 2.
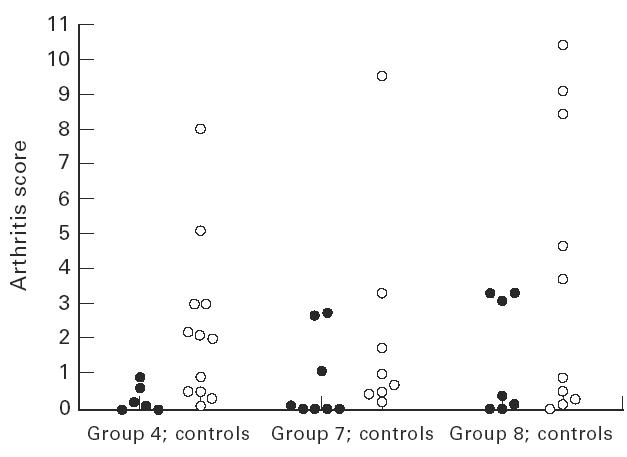
Results are shown in fuller detail for selected groups in which treatment with collagen peptide effectively suppressed development of arthritis. These were the groups 4 (short-term peptide treatment), groups 7 and 8 (long-term treatment) from Fig. 1 and their PBS controls. Points are average arthritis score for individual mice over 1–8 weeks after the booster injection with collagen.
Mice treated with 10 μg mouse CII had a lower mean arthritis score than the control groups. Administration of 10 μg of bovine CII had no effect on arthritis development.
Long-term intranasal administration of p707
Long-term treatment with the peptide p707 was more effective in obtaining suppression, both before and after disease induction. The dose of 10 μg per application was chosen on the basis of the one encouraging result obtained above. Groups of mice received 10 μg p707 twice per week intranasally, extending from 4 weeks prior to the first CII immunization until 8 weeks post-immunization (group 7). In a second experimental group of mice the same treatment was started after the second (boosting) injection and extended 8 weeks post-immunization (group 8). As before, the control groups received PBS alone. As shown in Fig. 1, the mean arthritis score in experimental group 7 (start of treatment before immunization) was strongly reduced compared with the control group (0.82 versus 2.18). Group 8, in which treatment was started post-immunization, also responded to the treatment, but the reduction in the arthritis score was less pronounced (1.45) compared with the control group. The results from these experiments are shown in detail in Fig. 2. The difference between treated and control groups was not quite significant for either of these treatments, P ≤ 0.065 and P ≤ 0.081, respectively, for groups 7 and 8 (Mann–Whitney); but when combined the difference became highly significant, P ≤ 0.015 (an even lower probability would be shown by considering the two treatments as independent and multiplying their P values).
Effect of nasal bovine CII treatment on chemiluminescence
Chemiluminescence was chosen as a non-invasive index of inflammation with which we are familiar [24,26]. It correlates with arthritis best early in the disease, so measurements were made on day 14 after the second booster injection. Mice were treated nasally with intact bovine CII at days −7, −5 and −3 relative to the first immunizing injection with varying doses of bovine CII. As can be seen in Fig. 3, suppression of the index was observed with a direct dose dependence.
Fig. 3.
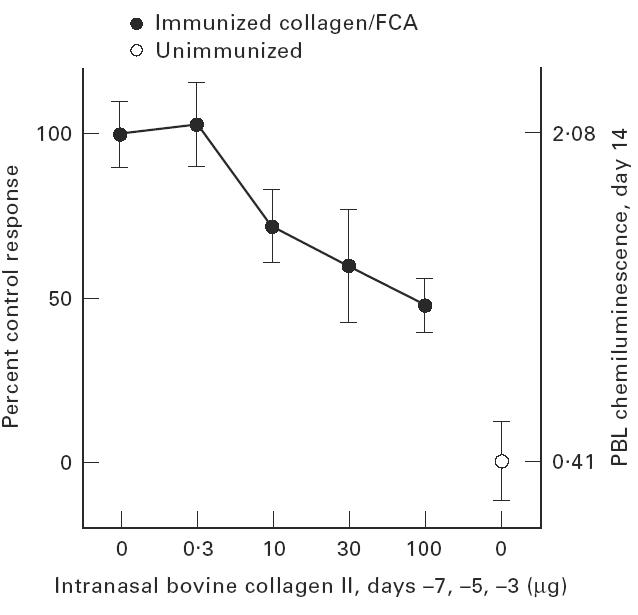
Effect of nasal tolerance on peripheral blood lymphocyte (PBL) chemiluminescence is shown for four groups of mice treated with varying doses of bovine type II collagen intranasally, or their PBS and unimmunized control groups (not the same as group 2 in Fig. 1).
DISCUSSION
The results of this study set clear limits on what can be expected of nasal administration of antigenic peptides as a disease-modulating treatment, at least as far as this particular animal model of autoimmunity is concerned. Little effect was obtained with short-term treatment. A single exceptional experiment did show a benefit, for reasons which are not understood, although the success of similar treatment is known to be sensitive to the cytokine environment at the time [27]. On the other hand, long-term treatment had a consistently beneficial effect. The neutrophil chemiluminescence assay here used as an index of inflammation also provided evidence of a beneficial effect.
The present investigation differs from previous studies performed in the mouse model of CIA, where treatment was with peptides from the immunodominant region of foreign collagen.
Khare et al. administered p250–270 of human CII (also identical with bovine and chicken CII) orally [28]. Myers et al. treated mice nasally with the slightly longer peptide 245–277 (in both peptides the core 260–270 is recognized by T cells) [6] at a higher dose (4 × 200 μg) than was used here. The homologous mouse sequence cross-reacts in vitro only at high peptide concentrations, and even then poorly [22]. Thus nasal tolerization with this peptide directly inhibited reactivity to the disease-inducing foreign CII, presumably without need for bystander suppression. Similarly, arthritis has been suppressed in the rat model by nasal administration of an immunodominant bovine CII epitope [16]. In contrast to these studies, p707 is a self epitope and appeared to act only through bystander suppression.
The treatment does not emerge from this study as one which is particularly effective. In this respect it is reminiscent of the discouraging experience with clinical trials of oral tolerance, where perhaps the main hope now is to find immunological parameters that would identify individual responsive patients [29]. A promising new approach is to search for ways of rousing the protective T cell population, perhaps by means of cytokines [27,30].
Acknowledgments
The authors would like to thank to Mrs Annette Hauschild for excellent assistance with the scoring of arthritic mice. This work was supported by the Senat of the City of Berlin.
REFERENCES
- 1.Weiner HL, Friedman A, Miller A, et al. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–37. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 2.Mitchison NA, Sieper J. Immunological basis of oral tolerance. Z-Rheumatol. 1995;54:141–4. [PubMed] [Google Scholar]
- 3.Weiner HL. Oral tolerance for the treatment of autoimmune diseases. Annu Rev Med. 1997;48:341–51. doi: 10.1146/annurev.med.48.1.341. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 5.Tian J, Atkinson MA, Clare Salzler M, Herschenfeld A, Forsthuber T, Lehmann PV, Laufman DV. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med. 1996;183:1561–7. doi: 10.1084/jem.183.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myers LK, Seyer JM, Stuart JM, Kang AH. Suppression of murine collagen-induced arthritis by nasal administration of collagen. Immunology. 1997;90:161–4. doi: 10.1046/j.1365-2567.1997.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderton SM, Burkhart C, Liu GY, Metzler B, Wraith DC. Antigen-specific tolerance induction and the immunotherapy of experimental autoimmune disease. CIBA Foundation Symposia. 1997 doi: 10.1002/9780470515525.ch9. in press. [DOI] [PubMed] [Google Scholar]
- 8.Whitacre CC, Gienapp IE, Orosz CG, Bitar DM. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J Immunol. 1991;147:2155–63. [PubMed] [Google Scholar]
- 9.Mitchison NA, O'Malley C. Three-cell-type clusters of T cells with antigen-presenting cells best explain the epitope linkage and noncognate requirements of the in vivo cytolytic response. Eur J Immunol. 1987;17:1579–83. doi: 10.1002/eji.1830171109. [DOI] [PubMed] [Google Scholar]
- 10.Stuhler G, Walden P. Collaboration helper cytotoxic T lymphocytes. Eur J Immunol. 1993;23:2279–86. doi: 10.1002/eji.1830230934. [DOI] [PubMed] [Google Scholar]
- 11.Stuhler G, Schlossman SF. Antigen organization regulates cluster formation and induction of cytotoxic T lymphocytes by helper T cell subsets. Proc Natl Acad Sci USA. 1997;94:622–7. doi: 10.1073/pnas.94.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cella M, Scheidegger D, Palmer Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller B, Mitchison NA. The importance of the back-signal from T cells into antigen-presenting cells in determining susceptibility to parasites. Phil Trans Roy Soc Lond B. 1997;352:1327–30. doi: 10.1098/rstb.1997.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel D, Wegmann DR. Protection of nonobese diabetic mice from diabetes by intranasal or subcutaneous administration of insulin peptide B-(9-23) Proc Natl Acad Sci USA. 1996;93:956–60. doi: 10.1073/pnas.93.2.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metzler B, Wraith DC. Inhibition of experimental autoimmune encephalomyelitis by inhalation but not oral administration of the encephalitogenic peptide: influence of MHC binding affinity. Int Immunol. 1993;5:1159–65. doi: 10.1093/intimm/5.9.1159. [DOI] [PubMed] [Google Scholar]
- 16.Staines NA, Harper N, Ward FJ, Malmström V, Holmdahl R, Bansal S. Mucosal tolerance and suppression of collagen-induced arthritis (CIA) induced by nasal inhalation of synthetic peptide 184–198 of bovine type II collagen (CII) expressing a dominant T cell epitope. Clin Exp Immunol. 1996;103:368–75. doi: 10.1111/j.1365-2249.1996.tb08289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prakken BJ, van der Zee R, Anderton SM, van Kooten PJ, Kuis W, van Eden W. Peptide-induced nasal tolerance for a mycobacterial heat shock protein 60 T cell epitope in rats suppresses both adjuvant arthritis and nonmicrobially induced experimental arthritis. Proc Natl Acad Sci USA. 1997;94:3284–9. doi: 10.1073/pnas.94.7.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Sabbagh A, Nelson PA, Akselband Y, Sobel RA, Weiner HL. Antigen-driven peripheral immune tolerance: suppression of experimental autoimmune encephalomyelitis and collagen-induced arthritis by aerosol administration of myelin basic protein or type II collagen. Cell Immunol. 1996;171:111–9. doi: 10.1006/cimm.1996.0180. [DOI] [PubMed] [Google Scholar]
- 19.Mauri C, Williams RO, Walmsley M, Feldmann M. Relationship between Th1/Th2 cytokine patterns and the arthritogenic response in collagen-induced arthritis. Eur J Immunol. 1996;26:1511–8. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- 20.Hesse M, Bayrak S, Mitchison A. Protective major histocompatibility complex genes and the role of interleukin-4 in collagen-induced arthritis. Eur J Immunol. 1996;26:3234–7. doi: 10.1002/eji.1830261259. [DOI] [PubMed] [Google Scholar]
- 21.Malmström V, Michaelsson E, Burkhardt H, Mattsson R, Vuorio E, Holmdahl R. Systemic versus cartilage-specific expression of a type II collagen-specific T-cell epitope determines the level of tolerance and susceptibility to arthritis. Proc Natl Acad Sci USA. 1996;93:4480–5. doi: 10.1073/pnas.93.9.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayrak S, Holmdahl R, Travers P, Lauster R, Hesse M, Dölling R, Mitchison NA. T cell response of I-Aq mice to self type II collagen: meshing of the binding motif of the I-Aq molecule with repetitive sequences results in autoreactivity to multiple epitopes. Int Immunol. 1997;11:1687–99. doi: 10.1093/intimm/9.11.1687. [DOI] [PubMed] [Google Scholar]
- 23.Miller ES. Purification of type II collagen by pepsin digestion. Biochemistry. 1972;11:4901. [Google Scholar]
- 24.Miesel R, Dietrich A, Ulbrich N, Kroeger H, Mitchison NA. Assessment of collagen type II induced arthritis in mice by whole blood chemiluminescence. Autoimmunity. 1994;19:153–9. doi: 10.3109/08916939408995690. [DOI] [PubMed] [Google Scholar]
- 25.Mitchison NA, Brunner MC. Association of H2Ab with resistance to collagen-induced arthritis in H2-recombinant mouse strains: an allele associated with reduction of several apparently unrelated responses. Immunogenetics. 1995;41:239–45. doi: 10.1007/BF00172065. [DOI] [PubMed] [Google Scholar]
- 26.Hauschild A, Kröger H, Mitchison NA, Ugrinovic S, Zwingenberger K. Thalidomide therapy of established collagen-induced arthritis not accompanied by an evident Th2 shift. Clin Exp Immunol. 1997;108:428–31. doi: 10.1046/j.1365-2249.1997.3781274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inobe JI, Chen Y, Weiner HL. In vivo administration of IL-4 induces TGF-beta-producing cells and protects animals from experimental autoimmune encephalomyelitis. Ann N Y Acad Sci. 1996;778:390–2. doi: 10.1111/j.1749-6632.1996.tb21153.x. [DOI] [PubMed] [Google Scholar]
- 28.Khare SD, Krco CJ, Griffiths MM, Luthra HS, David CS. Oral administration of an immunodominant human collagen peptide modulates collagen-induced arthritis. J Immunol. 1995;155:3653–9. [PubMed] [Google Scholar]
- 29.Gimsa U, Sieper JJB, Mitchison NA. Type II collagen serology: a guide to clinical responsiveness to oral tolerance. Int Rheumatol. 1997;16:237–40. doi: 10.1007/BF01375655. [DOI] [PubMed] [Google Scholar]
- 30.Gimsa U, Mitchison A. New perspectives on the Th1/Th2 paradigm. Current Opin Organ Transplant. 1997 in press. [Google Scholar]


