INTRODUCTION
Since its discovery as a biologically active molecule in the late 1980s, nitric oxide (NO) has been found to play an important role as signal molecule in many parts of the organism as well as cytotoxic or regulatory effector molecule of the innate immune response. The signal molecule NO is synthesized on demand for short periods of time (seconds to minutes) following enzyme activation of constitutively expressed endothelial NO synthase (eNOS) or neuronal NO synthase (nNOS). In contrast, the inducible NO synthase (iNOS) is expressed after cell activation only and then produces NO for comparatively long periods of time (hours to days). Thus, regulated short pulsative synthesis versus constant NO production differentiates between physiological and pathophysiological actions of NO (for review see [1]). As human monocytes in contrast to rodent ones do not produce large amounts of NO when activated in vitro, iNOS expression in human diseases has long been questionable. However, in the last 3 years data have accumulated on iNOS expression in a variety of human diseases or disorders. We here try to review our current understanding of the role of iNOS in human diseases.
MOLECULAR BIOLOGY OF HUMAN iNOS
The iNOS gene is under the transcriptional control of a variety of inflammatory mediators such as cytokines, lipopolysaccharide (LPS), and others (for review see [2]). iNOS cDNAs have independently been cloned from several tissues with only small differences in the deduced amino acid sequences [3–6]. The overall nucleotide sequence identity between human and murine iNOS cDNA is about 80% [3]. Molecular cloning revealed that the iNOS gene is about 37 kb in length [7] and is located on chromosome 17 at position 17cen-q11.2 [8]. The iNOS open reading frame is encoded by 27 exons, with translation initiation and termination in exons 2 and 27, respectively [9]. All intron/exon boundaries of the human iNOS gene conform strictly to the known GT/AG donor/acceptor rule. The structure of the coding region, especially of the cofactor binding sites, is very similar to those of human nNOS [10] and eNOS [11,12]. Southern blot analysis revealed single bands for nNOS and eNOS but multiple bands for iNOS exons 22–26 in humans and apes [13,14] when using the 3′ end of an iNOS cDNA probe. An unprocessed, highly mutated pseudogene has been localized on the same chromosomal region as the functional iNOS gene by fluorescence in situ hybridization (FISH) analysis [15]. Three minor allelic variants have been described [16–18], one bearing pathophysiological significance in resistance against malaria infection [18].
The high homology of iNOS isoforms among different species and various cell types suggests that they are all products of the same gene. However, human iNOS gene transcription in distinct cells is reported to be regulated differently. Significant differences between the human and the murine iNOS promoter region were found by 3′ analysis. Only 1.5 kb of the proximal 5′ flanking region of the murine promoter are necessary to confer inducibility to LPS and interferon-gamma (IFN-γ), whereas the human iNOS promoter is hyporesponsive to LPS/IFN-γ due to nucleotide exchanges in the LPS/IFN-γ-responsive enhancer region (−1083 to −1229) [19,20]. Additionally, the human transcription factor NF-κB, induced by treatment with IL-1β, tumour necrosis factor-alpha (TNF-α) and IFN-γ, binds to the iNOS promoter more weakly than mouse NF-κB does [21]. However, three regions with cytokine-responsive cis-regulatory elements (lying between −3.8 kb and −16 kb in the promoter region) confer cytokine inducibility [22,23]. Interestingly, the human iNOS gene contains a shear-stress responsive element (GAGACC) which is identical to that in human eNOS, but this element does not exist in the murine iNOS promoter [24]. Induction of NO production by this shear-stress element seems to be a key mediator for protection of cardiovascular diseases via inhibition of leucocyte adhesion, platelet aggregation, and vascular smooth muscle cell (VSMC) proliferation. At position -226 to -212 an element containing a sequence homology to the human hypoxia-responsive element (HRE) [25] was found in the murine promoter conferring iNOS induction at decreased oxygen tension in IFN-γ-activated macrophages [26]. Induction of the hypoxia-inducible factor-1 by hypoxia and binding to the iNOS-HRE in cooperation with IFN-γ leads to iNOS induction. The necessity for IFN-γ costimulation may help to limit iNOS expression to inflammatory sites with hypoxic conditions. Although the activity of the human HRE is questionable due to two mismatches in the consensus sequence, culture of human hepatoma cells under hypoxic conditions indeed led to iNOS induction [27]. In conclusion, the human iNOS promoter is one of the largest and most complex promoters known today (Fig. 1a, b), indicative of a tightly controlled iNOS gene expression.
Fig. 1.
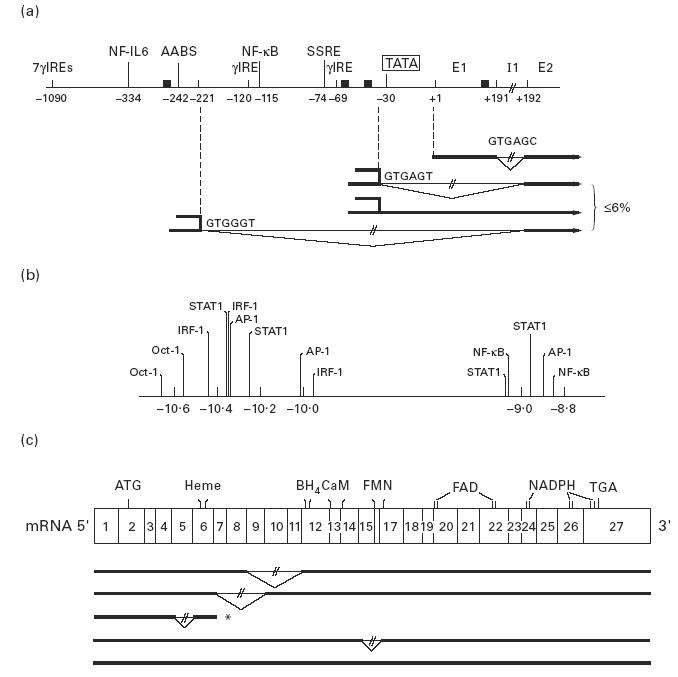
Schematic structure of the human inducible nitric oxide synthase (iNOS) 5′ flanking region (a), the upstream enhancer region (b), and the human iNOS mRNA (c). (a) The main transcriptional start site is denoted at position + 1. Several potential transcription factor binding sites are indicated. The TATA box begins at −30. TATA-independent iNOS transcripts have alternative splice sites at positions −221, −36 and +191 in the 5′ UTR of the gene. Possible start codons (▪) of open reading frames are located at −256, −65, −45, −40 and +187. (b) Structure of the distal part of the human iNOS promoter which seems to be a cytokine-responsive enhancer element. This promoter region (−10.9 to −8.7 kb) increases iNOS transcription orientation independently by a factor of 2. It contains multiple binding sites for transcription factors, which are activated in response to either IFN-γ (IRF-1, STAT1) or IL-1β (AP-1, IRF-1). (c) Alternative splicing of human iNOS mRNA. The ratios of alternatively to constitutively spliced mRNA differ among tissues and depend on activation by cytokines. *Truncated iNOS with exon 5 deletion abundant in human cerebellum [29]. γIRE, IFN-γ-responsive element; NF, nuclear factor; AABS, activator binding site; SSRE, shear stress responsive element; E, exon; I, intron; IRF, interferon regulatory factor; STAT, signal transducer and activator transcription.
A TATA box is located 30 bp upstream of the transcription start site and exon 2 contains the ATG initiation codon which lies in a Kozak consensus sequence, but about 6% of the cytokine-inducible iNOS transcripts in human macrophages and epithelial cells start at multiple transcription initiation sites (Fig. 1a), some extending several hundred base pairs upstream from the main TATA-directed initiation site [28]. Further diversity in the iNOS mRNAs is gained by alternative splicing. Five distinct alternative splicing regions have been found (Fig. 1c), one of which leads to the distinct deletion of exon 5 with a translational frame shift leading to a stop codon in exon 6 yielding a premature iNOS product of 134 amino acids. This deletion is abundant in cerebellum, suggesting a specific tissue-related function. Losses of exon 8 and 9, exon 9–11, or exon 15 and 16 by alternative splicing are in frame deletions [29]. Exon 15–16 deletion leads to iNOS proteins missing the FMN binding site. Alternative splicing of exon 1 together with the different transcription initiation sites leads to variable lengths of the 5′ untranslated region (UTR) in a minor fraction of the iNOS mRNA [28]. Human iNOS mRNA has a long and complex 5′UTR containing eight partially overlapping open reading frames prior to the start codon AUG. For other genes open reading frames in the 5′UTR of a specific RNA have been shown to inhibit its translation in a tissue-specific manner [30]. Moreover, the 3′UTRs in exon 27 of both human and murine iNOS mRNA also bear regulatory functions [31]. Both contain a UUAUUUAU motif that is common to a variety of cytokine and oncogene mRNAs [32]. This motif has been shown to confer RNA instability, resulting in rapid degradation [33] (thereby lowering basal promoter activity in transfection studies [34]). Comparison of the 3′ ends of the iNOS cDNAs from murine and human cells revealed poor sequence conservation within the 3′UTR except for these AU segments. The 3′UTR of murine iNOS mRNA contains two of these copies, while the human mRNA contains two additional elements. At least six nucleotides of these copies match the consensus motif. Rapid degradation due to the conserved AU-rich octanucleotide sequences results in transient expression of iNOS mRNA with a half life of about 6 h in murine cells. In the RAW 264 macrophage cell line two different 3′ ends have been found [35], indicating that mRNAs with different stabilities may be produced via alternative splicing. Exclusively in the human iNOS gene, the poly(A) signal, a GT-rich region, is located 10 bp downstream from the poly(A) site in the 3′ flanking region, while the usual poly(A) signal (AATAAA) is missing [34]. Differences in gene expression and mRNA stability due to these two distinct signals are still not yet known.
Obviously, further extensive studies are necessary to characterize all regulatory elements and transcription factors involved in transcriptional and post-transcriptional regulation of human iNOS gene expression. Current data indicate that its regulation reflects considerable complexity and tissue specificity.
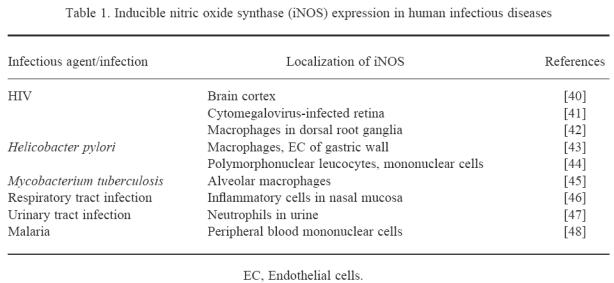
iNOS IN HUMAN INFECTIOUS DISEASES
In rodents, NO produced by activated macrophages via iNOS has been found to play a major role as antiparasitic cytotoxic effector molecule (for review see [36]). Although it is now established that human macrophages are able to express iNOS, the relevance of macrophage-produced NO in human infectious diseases still has to be elucidated (for reviews see [37–39]). Data concerning iNOS expression in human viral or bacterial infections are summarized in Table 1. In addition, in vitro killing via NO of Mycobacterium avium-intracellulare, Trypanosoma cruzi and Leishmania major by activated human macrophages has been found [49–51], as well as growth inhibition of Cryptococcus neoformans by activated human astrocytes [52]. Cytokine-activated human neutrophils contain the iNOS protein and mediate tyrosine nitration of ingested Staphylococcus aureus and Escherichia coli [53]. This proves iNOS expression during a variety of infectious diseases, but whether iNOS activity plays a dominant role in the combatting of pathogens in humans is still under debate.
Table 1.
There are good indications for a key defence role for NO at the interface between the human and the external environment. On the surface of the tongue facultative anaerobic bacteria reduce nitrate of the saliva rapidly to nitrite, which when swallowed will generate NO in the micromolar range due to the acidic conditions of the stomach [54]. Also, NO is continuously released from human skin surfaces. Patients on long-term antibiotic therapy show reduced NO generation, thus skin commensal bacteria are thought to reduce sweat nitrate to nitrite, which is subsequently reduced non-enzymatically to NO again due to the acidic conditions on the skin surface [55]. Interestingly, in nasal airways of healthy subjects iNOS has been found to be constitutively expressed (or continuously induced) apically in the epithelial cells of paranasal sinuses [56]. Sinus air contains NO in concentrations close to the highest permissible atmospheric pollution levels [57]. All these findings suggest that NO indeed plays a role in the human defence against invading pathogens.
Studies with animals have shown that increased NO production contributes to excessive vasodilation during endotoxic and cytokine-induced shock. In patients with septic shock, plasma NOx and nitrotyrosine concentrations are increased, and application of low doses of a specific NOS inhibitor partially reverses the widespread decrease in vascular tone as well as the fall in blood pressure, but it also produced a decrease in cardiac output (for reviews see [58,59]). However, additional data regarding iNOS expression in various organs, NO production by constitutive NOS versus iNOS, and differences in the role of NO in early versus late stages of shock are still necessary for understanding the role of iNOS activity in this disease.
iNOS IN HUMAN AUTOIMMUNE AND CHRONICALLY INFLAMMATORY DISEASES
Using immunocytochemistry, reverse transcriptase-polymerase chain reaction (RT-PCR), and in situ hybridization, iNOS expression has been described in rheumatoid arthritis (RA), multiple sclerosis (MS), and Sjögren's syndrome (Table 2). The NO oxidation product nitrite was found to be six- to 35-fold increased in the synovial fluid of RA patients compared with patients with osteoarthritis [89,90]. In active demyelinating lesions of MS patients, macrophages were found to stain for iNOS protein [63,64] and nitrotyrosine [91], indicative of nitrosative stress. These cells were found to produce high-output NO when isolated and cultured in vitro [64]. Type-1 diabetes, the most prevalent human immune-mediated disease, is the result of > 90% destruction of the pancreatic islet mass. Data concerning early human prediabetic stages do not exist, but excellent animal models (BB rats and NOD mice) are available which spontaneously develop diabetes closely resembling the human disease. In these animal models, iNOS protein has been detected in macrophage islet infiltrates during early disease stages [92,93]. Rat islet cells are extremely prone to NO-induced cell death [94–96]. At least part of the islet-specific toxicity of streptozotocin is due to intracellular release of its NO moiety [97], and streptozotocin is known to be extremely diabetogenic in humans as well. Isolated human islets are also lysed by NO, albeit at higher concentrations [98], and the human cells can also be activated to express iNOS mRNA and to produce NO in vitro [99–101]. All these data are suggestive of a similar role of iNOS activity in the human disease comparable to the animal models of diabetes. However, it has still to be shown whether inhibiting iNOS-derived NO in human patients will protect from tissue-destructive processes in RA, MS and type-1 diabetes.
Table 2.
iNOS expression has also been found in chronically inflammatory diseases of the airways, the vessels, the bowels, the kidney, the heart, the skin and the apex of teeth (Table 2). In these various diseases iNOS immunoreactivity has sometimes been localized to macrophages, but in most cases is found associated with epithelial cells around inflammatory foci. The role of epithelial iNOS activity is not really understood. The NO produced may either serve to limit bacterial invasion or may serve to limit local immune reactions and concomitant tissue destruction during Th1 immune responses. Local cytokine expression or cytokine response profiles in the relevant diseases positive for iNOS expression invariably correlate with the presence of proinflammatory Th1-type reactivities (Table 2). Of note is the association with IL-8, a cytokine usually labelled as chemoattractant, which on the ground of being a key inductor for iNOS expression in human keratinocytes [83] appears to exert proinflammatory activity in human diseases. Due to the close association of Th1-type cytokines and iNOS expression in human diseases, it could be argued that iNOS expression is an epiphenomenon of inflammatory diseases only. In this case iNOS expression would serve as an excellent marker for Th1 reactivity or imbalance. Future diagnostic evaluation may then use this one marker instead of measuring relative expressional levels of numerous cytokines.
iNOS EXPRESSION IN OTHER HUMAN DISORDERS
iNOS expression has been found in a variety of other human dis-orders (Table 3). However, courses of these diseases and cellular iNOS expression differ considerably.
Table 3.
Inducible nitric oxide synthase (iNOS) expression in human neurodegenerative diseases and heart infarction, during tumour development, after transplantation, during prostheses failure and myositis [79–91, 102–116]
iNOS and chronic neurodegeneration
iNOS protein was found post-mortem in the brains of patients with Alzheimer's and Parkinson's diseases [102–104]. Although neurones are highly susceptible to the cytotoxic action of NO, it is not yet clear whether iNOS expression accompanies late stages of disease or whether iNOS activity contributes to the course of these diseases.
iNOS and ischaemic events
As mentioned above, the human iNOS promoter contains a hypoxia-responsive element. Therefore it is not surprising that iNOS protein has been detected in cardiac myocytes and in infiltrating macrophages of patients several days after myocardial infarction [79,80,105]. In a rabbit heart infarction model, administration of specific NOS inhibitors significantly improved ventricular performance and increased myocardial blood flow in the surviving myocardium [117], suggesting tissue-destructive effects of NO production via iNOS. Moreover, after cardiac transplantation iNOS protein expression in myocytes and VSMC appears to correlate with contractile dysfunction [111].
Until now no data have been available concerning iNOS expression in human stroke. Data on animal stroke models imply that iNOS is expressed in areas of infarcted or injured brain. After focal cerebral ischaemia proinflammatory cytokines such as TNF-α, IL-1, and IL-6 are expressed, and neuronal tissue damage continues for days after the ischaemic event. Experimentally induced focal cerebral ischaemia in rats resulted in iNOS protein expression in neutrophils or in vascular cells 12–48 h after the ischaemic event, depending on the stroke model. In mutant mice deficient in nNOS or iNOS, infarct volume and neurological deficits were significantly smaller than those in normal mice. In contrast, eNOS knockout mice developed larger infarct volumes than the corresponding wild-type strains (for review see [118]). This proves the complexity of the NO-mediated effects, especially in brain, where high enzyme activities of eNOS (to maintain cerebral blood flow) and nNOS (to perform signalling functions) are normally present. Thus, low-output NO production by endothelial cells appears to promote regional cerebral blood flow during ischaemia, while neuronal NO production via nNOS and later long-lasting high-output production of NO via iNOS by inflammatory cells may be neurotoxic.
iNOS and tumours
The role of NO during tumour development also reveals a complex picture. High-output NO production by infiltrating macrophages can induce tumour cell cytostasis and/or cytotoxicity. iNOS protein has been detected in the vasculature, infiltrating macrophages, and tumour cells of human brain, breast, lung, and colon tumours (see Table 3). In rats, i.p. injection of colon adenocarcinoma cells was accompanied by a decrease in concanavalin A (Con A)-induced splenic T lymphocyte proliferation correlating with an increased NO production by splenic macrophages [119]. Low-output NO production within the tumour may increase tumour blood flow and promote angiogenesis [120]. In addition, NO produced by the tumour itself may inhibit proliferation or induce apoptosis of T lymphocytes, which would explain the suppression of host immune functions often observed to accompany tumour growth.
iNOS and transplantation/implantation
In recipients of myocardial allografts, iNOS protein was found in myocytes and VSMC. However, although iNOS mRNA was detected in all patients at some stage, this was episodic and occurred most frequently during the first 180 days after transplantation. It was postulated that iNOS expression and contractile dysfunction are causally related [111]. iNOS was also detected in various cell types involved in foreign body inflammatory reactions found around loosened joint replacement implants [113–115]. iNOS activity of activated and prosthetic wear debris-laden macrophages is likely to be noxious and may thus contribute to early prosthesis failure.
iNOS expression after noxious insults
iNOS mRNA was found in all skin biopsies taken from healthy volunteers treated with a single dose of either UV-A or UV-B, in contrast to untreated controls. iNOS protein was labelled in a band-like pattern confined to the highly proliferative basal layer of keratinocytes only [84]. iNOS expression was found to be maximal at 24 h, while 72 h post-irradiation none of the biopsies showed iNOS-specific signals, thus closely following the kinetics of erythema formation after sunburn which peaks at 24 h and lasts for 2 days. It appears as if iNOS activity is the cause of the observed long-lasting increase in local blood flow as well as erythema and oedema formation after prolonged sunlight exposure. In vitro, endogenous NO production via iNOS or exogenously added NO protected human dermal endothelial cells from UV-A-induced DNA fragmentation and subsequent apoptosis [121]. Thus, increased NO production may help to reduce UV-induced damage as a first indication of a protective role for iNOS expression in human skin. In animal models, NO plays a positive role in wound healing. Mice deficient in iNOS exhibit impaired wound healing, and iNOS gene transfer reverses the impaired healing of excisional wounds (see [122]).
The inflammatory response after traumatic brain injury (TBI) includes cytokine production, leucocyte infiltration, and microglial activation. In rats, 24 h and 48 h after experimentally induced TBI a marked peritrauma cerebrovascular iNOS protein expression was found predominantly in infiltrating neutrophils [123]. This suggests a role for iNOS activity in cerebrovascular disturbances and secondary brain injury after head trauma. Although data are not yet available, iNOS expression after TBI in man is most likely.
CONCLUDING REMARKS
Studies performed in rodents mostly imply that iNOS activity plays a detrimental role in experimental autoimmune or chronically inflammatory processes as well as in some other diseases. However, experimentally induced disorders are often constructed to show a maximal effect in a relatively short period of time. In addition, in many rodent strains Th1-associated reactions are favoured, whereas in humans an individually regulated balance between Th1- and Th2-mediated immune reactions is found. Thus, the question arises whether we can infer from animal (rodent) studies a role of iNOS in human diseases. It is crucial that we learn about the relative timing of disease onset and iNOS expression, especially as clinically overt disease often represents a relatively late-stage process. Another major problem in understanding the role of iNOS in human diseases is our lack of knowledge whether enzyme expression in vivo results in high- or low-output NO production. Although the human iNOS gene is more tightly regulated than the respective rodent gene (see first section), this does not necessarily imply low-output NO production in humans in all cases. Few reports only demonstrate high-output NO production by human cells in vitro, among these macrophages isolated from an MS lesion, hepatocytes, dermal endothelial cells, and polymorphonuclear leucocytes (PMNL) from periapical periodontitis patients, all producing high NO concentrations similar to activated rodent cells [64,124–126]. Thus, at least in vitro, human cells are indeed capable of high-output NO synthesis. Moreover, human cells appear to be more resistant to NO-mediated effects [127]. This may be due to a more effective DNA repair or to a higher capacity to induce protective mechanisms, e.g. expression of heat shock proteins (hsp). With rat islet cells over-expression of hsp70 has been shown to confer resistance against NO [128], and suppression of hsp70 by antisense RNA transfection of a human islet cell line confers susceptibility to NO-mediated cytotoxicity [129].
In summary, many data on human disorders with predominant proinflammatory Thl reactions involving activated macrophages and neutrophils point to a destructive activity of iNOS contributing to local tissue damage, but as pointed out earlier, iNOS expression is often found predominantly in cells of epithelial origin (see Table 2). We do not yet know the reason for this, but it may well be that in these cases NO serves as a protective agent limiting bacterial invasion or down-regulating local inflammatory reactions by inducing cytostasis and/or apoptosis in infiltrating immune cells like macrophages, neutrophils or T cells (for review see [1]). NO is known to affect gene regulation, e.g. via inhibition of NF-κB activation or by inhibiting the DNA-binding of NF-κB, AP-1 [1], and zinc finger transcription factors like Sp1 [130], thereby limiting Th1 reactions. Gene regulatory activities, instead of direct cytotoxic actions of iNOS-derived NO, may be of higher relevance in human diseases compared with experimentally induced rodent diseases (Fig. 2). Data are accumulating that NO not only induces but also inhibits programmed cell death induced by TNF-α or anti-CD95 (Fas/APO-1) [131,132]. We recently found that iNOS activity completely protects from UV-induced apoptosis in dermal endothelial cells [121]. A role for NO in protecting against adverse effects by reactive oxygen intermediates has also been postulated.
Fig. 2.
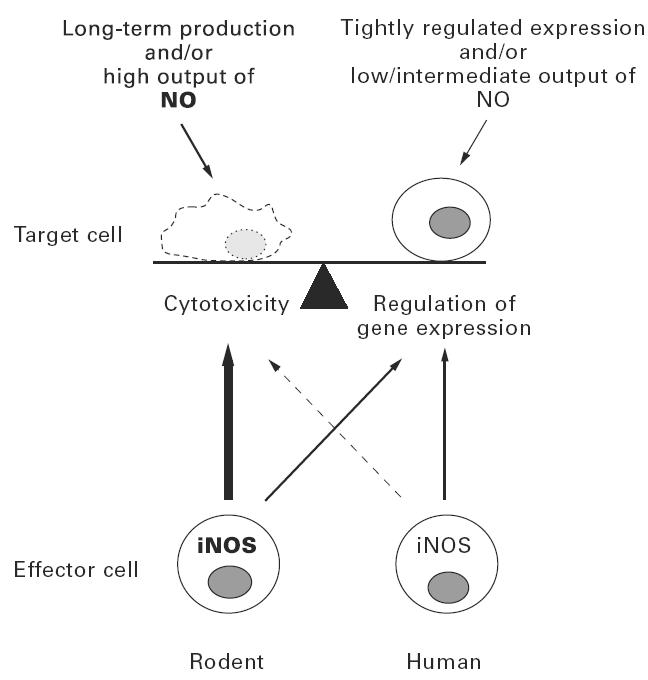
Current data suggest that inducible nitric oxide synthase (iNOS) expression and NO production in humans are much more tightly regulated than in rodents. In addition, human cells appear to be less susceptible to NO than rodent cells. Thus, in humans NO-mediated gene regulatory effects may be of more relevance than cytotoxic effects.
In conclusion, a large amount of data concerning iNOS expression in a variety of human diseases has accumulated, but we are still far from understanding the precise role of iNOS activity in most of these diseases.
Acknowledgments
The authors would like to thank Dr H. Kolb for helpful discussions.
References
- 1.Kröncke KD, Fehsel K, Kolb-Bachofen V. Nitric oxide: cytotoxicity versus cytoprotection. How, why, when where? NO Biol Chem. 1997;1:107–20. doi: 10.1006/niox.1997.0118. [DOI] [PubMed] [Google Scholar]
- 2.Kröncke KD, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase and its product nitric oxide, a small molecule with complex biological activities. Biol Chem. 1995;376:327–43. doi: 10.1515/bchm3.1995.376.6.327. [DOI] [PubMed] [Google Scholar]
- 3.Geller DA, Lowenstein CR, Shapiro RA, et al. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci USA. 1993;90:3491–5. doi: 10.1073/pnas.90.8.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles IG, Palmer RM, Hickery MS, Bayliss MT, Chubb AP, Hall VS, Moss DW, Moncada S. Cloning, characterization, and expression of a cDNA encoding an inducible nitric oxide synthase from the human chondrocyte. Proc Natl Acad Sci USA. 1993;90:11419–23. doi: 10.1073/pnas.90.23.11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherman PA, Laubach VE, Reep BR, Wood ER. Purification and cDNA sequence of an inducible nitric oxide synthase from a human tumor cell line. Biochemistry. 1993;32:11600–5. doi: 10.1021/bi00094a017. [DOI] [PubMed] [Google Scholar]
- 6.Maier R, Bilbe G, Rediske J, Lotz M. Inducible nitric oxide synthase from human articular chondrocytes: cDNA cloning and analysis of mRNA expression. Biochim Biophys Acta. 1994;1208:145–50. doi: 10.1016/0167-4838(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 7.Chartrain NA, Geller DA, Koty PP, et al. Molecular cloning, structure, and chromosomal localization of the human inducible nitric oxide synthase gene. J Biol Chem. 1994;269:6765–72. [PubMed] [Google Scholar]
- 8.Marsden PA, Heng HHG, Duff CL, Shi XM, Tsui LC, Hall AV. Localization of the human gene for inducible nitric oxide synthase (NOS2) to chromosome 17q11.2-q12. Genomics. 1994;19:183–5. doi: 10.1006/geno.1994.1039. [DOI] [PubMed] [Google Scholar]
- 9.Xu W, Charles IG, Liu L, Moncada S, Emson P. Molecular cloning and structural organization of the inducible nitric oxide synthase gene (NOS2) Biochem Biophys Res Commun. 1996;219:784–8. doi: 10.1006/bbrc.1996.0311. [DOI] [PubMed] [Google Scholar]
- 10.Hall AV, Antoniou H, Wang Y, et al. Structural organization of the human neuronal nitric oxide synthase gene (NOS1) J Biol Chem. 1994;269:33082–90. [PubMed] [Google Scholar]
- 11.Marsden PA, Heng HHQ, Scherer SW, Stewart RJ, Hall AV, Shi XM, Tsui LC, Schappert KT. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem. 1993;268:17478–88. [PubMed] [Google Scholar]
- 12.Nadaud S, Bonnardeaux A, Lathrop M, Soubrier F. Gene structure, polymorphism and mapping of the human endothelial nitric oxide synthase gene. Biochem Biophys Res Commun. 1994;198:1027–33. doi: 10.1006/bbrc.1994.1146. [DOI] [PubMed] [Google Scholar]
- 13.Xu W, Charles IG, Liu L, Koni PA, Moncada S, Emson P. Molecular genetic analysis of the duplication of human inducible nitric oxide synthase (NOS2) sequences. Biochem Biophys Res Commun. 1995;212:466–72. doi: 10.1006/bbrc.1995.1993. [DOI] [PubMed] [Google Scholar]
- 14.Bloch KD, Wolfram JR, Brown DM, et al. Three members of the nitric oxide synthase II gene family (NOS2A, NOS2B, and NOS2C) colocalize to human chromosome 17. Genomics. 1995;27:526–30. doi: 10.1006/geno.1995.1086. [DOI] [PubMed] [Google Scholar]
- 15.Park CS, Lee HS, Lee HY, Krishna G. An unprocessed pseudogene of inducible nitric oxide synthase gene in human. NO Biol Chem. 1997;1:294–300. doi: 10.1006/niox.1997.0138. [DOI] [PubMed] [Google Scholar]
- 16.Esumi H, Ogura T, Kurashima Y, Adachi H, Hokari A, Weisz A. Implication of nitric oxide synthase in carcinogenesis: analysis of the human inducible nitric oxide synthase gene. Pharmacogenetics. 1995;5:166–70. doi: 10.1097/00008571-199512001-00021. [DOI] [PubMed] [Google Scholar]
- 17.Bellamy R, Hill AVS. Short report on DNA marker at candidate locus: a bi-allelic tetranucleotide repeat in the promoter of the human inducible nitric oxide synthase gene. Clin Genet. 1997;52:192–3. doi: 10.1111/j.1399-0004.1997.tb02544.x. [DOI] [PubMed] [Google Scholar]
- 18.Kun FJ, Mordmüller B, Lell B, Lehman LG, Luckner D, Kremsner PG. Polymorphism in promoter region of inducible nitric oxide synthase gene and protection against malaria. Lancet. 1998;351:265–6. doi: 10.1016/S0140-6736(05)78273-8. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Laubach VE, Alley EW, Edwards KA, Sherman PA, Russell SW, Murphy WJ. Transcriptional basis for hyporesponsiveness of the human inducible nitric oxide synthase gene to lipopolysaccharide/interferon-γ. J Leukocyte Biol. 1996;59:575–85. doi: 10.1002/jlb.59.4.575. [DOI] [PubMed] [Google Scholar]
- 20.Spitsin SV, Farber JL, Bertovich M, Moehren G, Koprowski H, Michaels FH. Human- and mouse-inducible nitric oxide synthase promoters require activation of phosphatidylcholine-specific phospholipase C and NF-κB. Mol Med. 1997;3:315–26. [PMC free article] [PubMed] [Google Scholar]
- 21.Kolyada AY, Savikovsky N, Madias NF. Transcriptional regulation of the human iNOS gene in vascular-smooth-muscle cells and macrophages: evidence for tissue-specificity. Biochem Biophys Res Commun. 1996;220:600–5. doi: 10.1006/bbrc.1996.0449. [DOI] [PubMed] [Google Scholar]
- 22.De Vera ME, Shapiro RA, Nussler AK, Mudgett JS, Simmons RL, Morris SM, Billiar TR, Geller DA. Transcriptional regulation of human inducible nitric oxide synthase (NOS2) gene by cytokines: initial analysis of the human NOS2 promoter. Proc Natl Acad Sci USA. 1996;93:1054–9. doi: 10.1073/pnas.93.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linn SC, Morelli PJ, Edry I, Cottongim SE, Szabó C, Salzman AL. Transcriptional regulation of human inducible nitric oxide synthase gene in an intestinal epithelial cell line. Am J Physiol. 1997;272:G1499–508. doi: 10.1152/ajpgi.1997.272.6.G1499. [DOI] [PubMed] [Google Scholar]
- 24.Nunokawa Y, Ishida N, Tanaka S. Promoter analysis of human inducible nitric oxide synthase gene associated with cardiovascular homeostasis. Biochem Biophys Res Commun. 1994;200:802–7. doi: 10.1006/bbrc.1994.1522. [DOI] [PubMed] [Google Scholar]
- 25.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1994;12:5447–54. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melillo G, Musso T, Sica A, Taylor LS, Cox GW, Varesio L. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase pathway. J Exp Med. 1995;182:1683–93. doi: 10.1084/jem.182.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshioka K, Thompson J, Miller MJS, Fisher JW. Inducible nitric oxide synthase expression and erythropoietin production in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 1997;232:702–6. doi: 10.1006/bbrc.1997.6323. [DOI] [PubMed] [Google Scholar]
- 28.Chu SC, Wu HP, Banks TC, Eissa T, Moss J. Structural diversity in the 5′ untranslated region of cytokine-stimulated human inducible nitric oxide synthase mRNA. J Biol Chem. 1995;270:10625–30. doi: 10.1074/jbc.270.18.10625. [DOI] [PubMed] [Google Scholar]
- 29.Eissa NT, Strauss AJ, Haggerty CM, Choo EK, Chu SC, Moss J. Alternative splicing of human inducible nitric-oxide synthase mRNA. J Biol Chem. 1996;271:27184–7. doi: 10.1074/jbc.271.43.27184. [DOI] [PubMed] [Google Scholar]
- 30.Zimmer A, Zimmer AM, Reynolds K. Tissue specific expression of the retinoic acid receptor-β2: regulation by short open reading frames in the 5′-noncoding region. J Cell Biol. 1994;127:1111–9. doi: 10.1083/jcb.127.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans T, Carpenter A, Cohen J. Inducible nitric-oxide-synthase mRNA is transiently expressed and destroyed by a cycloheximide-sensitive process. Eur J Biochem. 1994;219:563–9. doi: 10.1111/j.1432-1033.1994.tb19972.x. [DOI] [PubMed] [Google Scholar]
- 32.Caput D, Beutler B, Hartog K, Thayer R, Brown-Shimer S, Cerami A. Identification of a common nucleotide sequence in the 3′-translated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci USA. 1986;83:1670–4. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–67. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 34.Nunokawa Y, Oikawa S, Tanaka S. Expression of human inducible nitric oxide synthase is regulated by both promoter and 3′-regions. Biochem Biophys Res Commun. 1997;233:523–6. doi: 10.1006/bbrc.1997.6471. [DOI] [PubMed] [Google Scholar]
- 35.Xie QW, Cho HJ, Calaycay J, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–8. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 36.James SL. Role of nitric oxide in parasitic infections. Microbiol Rev. 1995;59:533–47. doi: 10.1128/mr.59.4.533-547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denis M. Human monocytes/macrophages: NO or no NO? J Leukocyte Biol. 1994;55:682–4. doi: 10.1002/jlb.55.5.682. [DOI] [PubMed] [Google Scholar]
- 38.Schoendon G, Schneemann M, Walter R, Blau N, Hofer S, Schaffner A. Nitric oxide and infection: another view. Clin Infect Dis. 1995;21(Suppl. 2):S152–7. doi: 10.1093/clinids/21.supplement_2.s152. [DOI] [PubMed] [Google Scholar]
- 39.Albina JE. On the expression of nitric oxide synthase by human macrophages. Why no NO? J Leukocyte Biol. 1995;58:643–9. doi: 10.1002/jlb.58.6.643. [DOI] [PubMed] [Google Scholar]
- 40.Adamson DC, Wildemann B, Sasaki M, Glass JD, McArthur JC, Christov VI, Dawson TM, Dawson VL. Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science. 1996;274:1917–21. doi: 10.1126/science.274.5294.1917. [DOI] [PubMed] [Google Scholar]
- 41.Dighiero P, Reux I, Hauw JJ, Fillet AM, Courtois Y, Goureau O. Expression of inducible nitric oxide synthase in cytomegalovirus-infected glial cells of retinas from AIDS patients. Neuroscience Letters. 1994;166:31–34. doi: 10.1016/0304-3940(94)90833-8. [DOI] [PubMed] [Google Scholar]
- 42.Nagano I, Shapshak P, Yoshioka M, Xin K, Nakamura S, Bradley WG. Increased NADPH-diaphorase reactivity and cytokine expression in dorsal root ganglia in acquired immunodeficiency syndrome. J Neurol Sci. 1996;136:117–28. doi: 10.1016/0022-510x(95)00317-u. [DOI] [PubMed] [Google Scholar]
- 43.Stachura J, Konturek JW, Karczewska A, Domschke W, Popiela T, Konturek SJ. Helicobacter pylori from duodenal ulcer patients expresses inducible nitric oxide synthase immunoreactivity in vivo and in vitro. J Physiol Pharmacol. 1996;47:131–5. [PubMed] [Google Scholar]
- 44.Mannick EE, Bravo LE, Zarama G, et al. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238–43. [PubMed] [Google Scholar]
- 45.Nicholson S, Bonecini-Almeida MG, Lapa e Silva JR, et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furukawa K, Harrison DG, Saleh D, Shennib H, Chagnon FP, Giaid A. Expression of nitric oxide synthase in the human nasal mucosa. Am J Respir Crit Care Med. 1996;153:847–50. doi: 10.1164/ajrccm.153.2.8564142. [DOI] [PubMed] [Google Scholar]
- 47.Wheeler MA, Smith SD, Garcia-Cardeña G, Nathan CF, Weiss RM, Sessa WC. Bacterial infection induces nitric oxide synthase in human neutrophils. J Clin Invest. 1997;99:110–6. doi: 10.1172/JCI119121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anstey NM, Weinberg JB, Hassanali MY, et al. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184:557–67. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukocyte Biol. 1991;49:380–7. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- 50.Muñoz-Fernández MA, Fernández MA, Fresno M. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-α and IFN-γ through a nitric oxide-dependent mechanism. Immunol Letters. 1992;33:35–40. doi: 10.1016/0165-2478(92)90090-b. [DOI] [PubMed] [Google Scholar]
- 51.Vouldoukis I, Riveros-Moreno V, Dugas B, Ouaaz F, Bécherel P, Debré P, Moncada S, Mossalayi MD. The killing of Leishmania major by human macrophages is mediated by nitric oxide induced after ligation ot the FcεRII/CD23 surface antigen. Proc Natl Acad Sci USA. 1995;92:7804–8. doi: 10.1073/pnas.92.17.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee SC, Dickson DW, Brosnan CF, Casadevall A. Human astrocytes inhibit Cryptococcus neoformans growth by a nitric oxide-mediated mechanism. J Exp Med. 1994;180:365–9. doi: 10.1084/jem.180.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans TJ, Buttery LDK, Carpenter A, Springall DR, Cohen J. Cytokine-treated human neutrophils contain inducible nitric oxide synthase that produces nitration of ingested bacteria. Proc Natl Acad Sci USA. 1996;93:9553–8. doi: 10.1073/pnas.93.18.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benjamin N, O'Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 55.Weller R, Pattullo S, Smith L, Golden M, Ormerod A, Benjamin N. Nitric oxide is generated on the skin surface by reduction of sweat nitrate. J Invest Dermatol. 1996;107:327–31. doi: 10.1111/1523-1747.ep12363167. [DOI] [PubMed] [Google Scholar]
- 56.Guo FH, de Raeve HR, Rice TW, Stuehr DJ, Thunnissen Fbjm, Erzurum SC. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci USA. 1996;92:7809–13. doi: 10.1073/pnas.92.17.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lundberg JON, Farkas-Szallasi T, Weitzberg E, Rinder J, Lidholm J, Änggård A, Lundberg JM, Alving K. High nitric oxide production in human paranasal sinuses. Nature Med. 1995;1:370–3. doi: 10.1038/nm0495-370. [DOI] [PubMed] [Google Scholar]
- 58.Cobb JP, Danner RL. Nitric oxide and septic shock. JAMA. 1996;275:1192–6. [PubMed] [Google Scholar]
- 59.Thiemermann C. Nitric oxide and septic shock. Gen Pharmac. 1997;29:159–66. doi: 10.1016/s0306-3623(96)00410-7. [DOI] [PubMed] [Google Scholar]
- 60.Sakurai H, Kohsaka H, Liu MF, Higashiyama H, Hirata Y, Kanno K, Saito I, Miyasaka N. Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J Clin Invest. 1995;96:2357–63. doi: 10.1172/JCI118292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McInnes IB, Leung BP, Field M, et al. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med. 1996;184:1519–24. doi: 10.1084/jem.184.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grabowski PS, Wright PK, van't Hof RJ, Helfrich MH, Oshima H, Ralston SH. Immunolocalization of inducible nitric oxide synthase in synovium and cartilage in rheumatoid arthritis and osteoarthritis. Br J Rheumatol. 1997;36:651–5. doi: 10.1093/rheumatology/36.6.651. [DOI] [PubMed] [Google Scholar]
- 63.Bagasra O, Michaels FH, Zheng YM, Bobroski LE, Spitsin SV, Fu ZF, Tawadros R, Koprowski H. Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc Natl Acad Sci USA. 1995;92:12041–5. doi: 10.1073/pnas.92.26.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeGroot CJA, Ruuls SR, Theeuwes JWM, Dukstra CD, van der Valk P. Immunocytochemical characterization of the expression of inducible and constitutive isoforms of nitric oxide synthase in demyelinating multiple sclerosis lesions. J Neuropathol Exp Neurol. 1997;56:10–20. doi: 10.1097/00005072-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 65.Konttinen YT, Platts LAM, Tuominen S, et al. Role of nitric oxide in Sjögren's syndrome. Arthritis Rheum. 1997;40:875–83. doi: 10.1002/art.1780400515. [DOI] [PubMed] [Google Scholar]
- 66.Hamid Q, Springall DR, Riveros-Moreno V, et al. Induction of nitric oxide synthase in asthma. Lancet. 1993;342:1510–3. doi: 10.1016/s0140-6736(05)80083-2. [DOI] [PubMed] [Google Scholar]
- 67.Tracey WR, Xue C, Klinghofer V, Barlow J, Pollock JS, Förstermann U, Johns RA. Immunochemical detection of inducible NO synthase in human lung. Am J Physiol. 1994;266:L722–7. doi: 10.1152/ajplung.1994.266.6.L722. [DOI] [PubMed] [Google Scholar]
- 68.Saleh D, Barnes PJ, Giaid A. Increased production of the potent oxidant peroxynitrite in the lungs of patients with idiopathic pulmonary fibrosis. Am J Crit Care Med. 1997;155:1763–9. doi: 10.1164/ajrccm.155.5.9154889. [DOI] [PubMed] [Google Scholar]
- 69.Buttery LDK, Springall DR, Chester AH, Evans TJ, Stanfield N, Parums DV, Yacoub MH, Polak JM. Inducible nitric oxide synthase is present within human atherosclerotic lesions and promotes the formation and activity of peroxynitrite. Lab Invest. 1996;75:77–85. [PubMed] [Google Scholar]
- 70.Lafond-Walker A, Chen CL, Augustine S, Wu TC, Hruban RH, Lowenstein CJ. Inducible nitric oxide synthase expression in coronary arteries of transplanted human hearts with accelerated graft arteriosclerosis. Am J Pathol. 1997;151:919–25. [PMC free article] [PubMed] [Google Scholar]
- 71.Mourelle M, Casellas F, Guarner F, Salas A, Riveros-Moreno V, Moncada S, Malagelada JR. Induction of nitric oxide synthase in colonic smooth muscle from patients with toxic megacolon. Gastroenterology. 1995;109:1497–502. doi: 10.1016/0016-5085(95)90636-3. [DOI] [PubMed] [Google Scholar]
- 72.Godkin AJ, de Belder AJ, Villa L, Wong A, Beesley JE, Kane SP, Martin JF. Expression of nitric oxide synthase in ulcerative colitis. Eur J Clin Invest. 1996;26:867–72. doi: 10.1111/j.1365-2362.1996.tb02131.x. [DOI] [PubMed] [Google Scholar]
- 73.Singer II, Kawa DW, Scott S, Weidener RJ, Mumford RA, Riehl TE, Stenson WF. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996;111:871–85. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- 74.Ikeda I, Kasajima T, Ishijama S, Shimojo T, Takeo Y, Nishikawa S, Hiroe M, Mitsunaga A. Distribution of inducible nitric oxide synthase in ulcerative colitis. Am J Gastroenterol. 1997;92:1339–41. [PubMed] [Google Scholar]
- 75.Levine JJ, Pettei MJ, Valderrama E, Gold DM, Kessler BH, Trachtman H. Nitric oxide and inflammatory bowel disease: evidence for local intestinal production in children with active colonic disease. J Pediatr Gastr Nutr. 1998;26:34–38. doi: 10.1097/00005176-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 76.Ford H, Watkins S, Reblock K, Rowe M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 1997;32:275–82. doi: 10.1016/s0022-3468(97)90194-9. [DOI] [PubMed] [Google Scholar]
- 77.ter Steege J, Buurman W, Arends JW, Forget P. Presence of inducible nitric oxide synthase, nitrotyrosine, CD68, and CD14 in the small intestine in celiac disease. Lab Invest. 1997;77:29–36. [PubMed] [Google Scholar]
- 78.Kashem A, Endoh M, Yano N, Yamauchi F, Nomoto Y, Sakai H. Expression of inducible-NOS in human glomerulonephritis: the possible source is infiltrating monocytes/macrophages. Kidney Int. 1996;50:392–9. doi: 10.1038/ki.1996.328. [DOI] [PubMed] [Google Scholar]
- 79.Haywood GA, Tsao PS, von der Leyen HE, et al. Expression of inducible nitric oxide synthase in human heart failure. Circulation. 1996;93:1087–94. doi: 10.1161/01.cir.93.6.1087. [DOI] [PubMed] [Google Scholar]
- 80.Satoh M, Nakamura M, Tamura G, Makita S, Segawa I, Tashiro A, Satodate R, Hiramori K. Inducible nitric oxide synthase and tumor necrosis factor-alpha in myocardium in human dilated cardiomyopathy. J Am Coll Cardiol. 1997;29:716. doi: 10.1016/s0735-1097(96)00567-0. [DOI] [PubMed] [Google Scholar]
- 81.Adams V, Yu J, Möbius-Winkler S, Linke A, Weigl C, Hilbrich L, Schuler G, Hambrecht R. Increased inducible nitric oxide synthase in skeletal muscle biopsies from patients with chronic heart failure. Biochem Mol Med. 1997;61:152–60. doi: 10.1006/bmme.1997.2598. [DOI] [PubMed] [Google Scholar]
- 82.Habib FM, Springall DR, Davies GJ, Oakley CM, Yacoub MH, Polak JM. Tumor necrosis factor and inducible nitric oxide synthase in dilated cardiomyopathy. Lancet. 1996;347:1151–5. doi: 10.1016/s0140-6736(96)90610-8. [DOI] [PubMed] [Google Scholar]
- 83.Bruch-Gerharz D, Fehsel K, Suschek C, Michel G, Ruzicka T, Kolb-Bachofen V. A proinflammatory activity of interleukin 8 in human skin: expression of the inducible nitric oxide synthase in psoriatic lesions and cultured keratinocytes. J Exp Med. 1996;184:2007–12. doi: 10.1084/jem.184.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuhn A, Fehsel K, Lehmann P, Krutmann J, Ruzicka T, Kolb-Bachofen V. Aberrant timing in epidermal expression of inducible nitric oxide synthase after UV irradiation in cutaneous lupus erythematosus. J Invest Dermatol. 1998;111:105–5. doi: 10.1046/j.1523-1747.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- 85.Belmont HM, Levartovsky D, Goel A, Amin A, Giorno R, Rediske J, Skovron ML, Abramson SB. Increased nitric oxide production accompanied by the up-regulation of inducible nitric oxide synthase in vascular endothelium from patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:1810–6. doi: 10.1002/art.1780401013. [DOI] [PubMed] [Google Scholar]
- 86.Yamamoto T, Katayama I, Nishioka K. Nitric oxide production and inducible nitric oxide synthase expression in systemic sclerosis. J Rheumatol. 1998;25:314–7. [PubMed] [Google Scholar]
- 87.Rowe A, Farrell AM, Bunker CB. Constitutive endothelial and inducible nitric oxide synthase in inflammatory dermatoses. Br J Dermatol. 1997;136:18–23. [PubMed] [Google Scholar]
- 88.Takeichi O, Saito I, Hayashi M, Tsurumachi T, Saito T. Production of human-inducible nitric oxide synthase in radicular cysts. J Endodont. 1998;24:157–60. doi: 10.1016/S0099-2399(98)80173-8. [DOI] [PubMed] [Google Scholar]
- 89.Farrell AJ, Blake DR, Palmer RMJ, Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis. 1992;51:1219–22. doi: 10.1136/ard.51.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ueki Y, Miyake S, Tominaga Y, Eguchi K. Increased nitric oxide levels in patients with rheumatoid arthritis. J Rheumatol. 1996;23:230–6. [PubMed] [Google Scholar]
- 91.Hooper DC, Bagasra O, Marini JC, et al. Prevention of experimental allergic encephalomyelitis by targeting nitric oxide and peroxynitrite: implications for the treatment of multiple sclerosis. Proc Natl Acad Sci USA. 1997;94:2528–33. doi: 10.1073/pnas.94.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kleemann R, Rothe H, Kolb-Bachofen V, Xie QW, Nathan C, Martin S, Kolb H. Transcription and translation of inducible nitric oxide synthase in the pancreas of prediabetic BB rats. FEBS Letters. 1993;328:9–12. doi: 10.1016/0014-5793(93)80954-s. [DOI] [PubMed] [Google Scholar]
- 93.Rothe H, Faust A, Schade U, Kleemann R, Bosse G, Hibino T, Martin S, Kolb H. Cyclophosphamide treatment of female non-obese diabetic mice causes enhanced expression of inducible nitric oxide synthase and interferon-gamma, but not of interleukin-4. Diabetologia. 1994;37:1154–8. doi: 10.1007/BF00418380. [DOI] [PubMed] [Google Scholar]
- 94.Kröncke KD, Kolb-Bachofen V, Berschick B, Burkart V, Kolb H. Activated macrophages kill pancreatic syngeneic islet cells via arginine-dependent nitric oxide generation. Biochem Biophys Res Commun. 1991;175:752–8. doi: 10.1016/0006-291x(91)91630-u. [DOI] [PubMed] [Google Scholar]
- 95.Steiner L, Kröncke KD, Fehsel K, Kolb-Bachofen V. Endothelial cells as cytotoxic effector cells: cytokine-activated islet endothelial cells lyse syngeneic islet cells via nitric oxide. Diabetologia. 1997;40:150–5. doi: 10.1007/s001250050656. [DOI] [PubMed] [Google Scholar]
- 96.Kröncke KD, Brenner H, Rodriguez ML, Etzkorn K, Noack EA, Kolb H, Kolb-Bachofen V. Pancreatic islet cells are highly susceptible towards the cytotoxic effects of chemically generated nitric oxide. Biochem Biophys Acta. 1993;1182:221–9. doi: 10.1016/0925-4439(93)90144-p. [DOI] [PubMed] [Google Scholar]
- 97.Kröncke KD, Fehsel K, Sommer A, Rodriguez ML, Kolb-Bachofen V. Nitric oxide generation during cellular metabolization of the diabetogenic N-methyl-N-nitroso-urea streptozotocin contributes to islet cell DNA damage. Biol Chem. 1995;376:179–85. doi: 10.1515/bchm3.1995.376.3.179. [DOI] [PubMed] [Google Scholar]
- 98.Eizirik DL, Delaney CA, Green MHL, Cunningham JM, Thorpe JR, Pipeleers DG, Hellerstöm C, Green IC. Nitric oxide donors decrease the function and survival of human pancreatic islets. Mol Cell Endocrinol. 1996;118:71–83. doi: 10.1016/0303-7207(96)03768-9. [DOI] [PubMed] [Google Scholar]
- 99.Eizirik DL, Sandler S, Welsh N, et al. Cytokines suppress human islet function irrespective of their effects on nitric oxide generation. J Clin Invest. 1994;93:1968–74. doi: 10.1172/JCI117188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Corbett JA, Sweetland MA, Wang JL, Lancaster JR, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci USA. 1993;90:1731–5. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vara E, Arias-Diaz J, Garcia C, Hernández J, Garcia-Carreras C, Cuadrado A, Balibrea JL. Production of TNFα, IL-1, IL-6 and nitric oxide by isolated human islets. Transplant Proc. 1995;27:3367–71. [PubMed] [Google Scholar]
- 102.Vodovotz Y, Lucia MS, Flanders KC, et al. Inducible nitric oxide synthase in tangle-bearing neurons of patients with Alzheimer's disease. J Exp Med. 1996;184:1425–33. doi: 10.1084/jem.184.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wallace MN, Geddes JG, Farquhar DA, Masson MR. Nitric oxide synthase in reactive astrocytes adjacent to β-amyloid plaques. Exp Neurol. 1997;144:266–72. doi: 10.1006/exnr.1996.6373. [DOI] [PubMed] [Google Scholar]
- 104.Hunot S, Boissière F, Faucheux B, Brugg B, Mouatt-Prient A, Agid Y, Hirsch EC. Nitric oxide synthase and neuronal vulnerability in Parkinson's disease. Neuroscience. 1996;72:355–63. doi: 10.1016/0306-4522(95)00578-1. [DOI] [PubMed] [Google Scholar]
- 105.Wildhirt SM, Dudek RR, Suzuki H, Narayan KS, Winder S, Choe J, Bing JR. Expression of nitric oxide synthase isoforms after myocardial infarctions in humans. Endothelium. 1995;3:209–24. [Google Scholar]
- 106.Cobbs CS, Brenman JE, Aldape KD, Bredt DS, Israel MA. Expression of nitric oxide synthase in human central nervous system tumors. Cancer Res. 1995;55:727–30. [PubMed] [Google Scholar]
- 107.Thomsen LL, Miles DW, Happerfield L, Bobrow LG, Knowles RG, Moncada S. Nitric oxide synthase activity in human breast cancer. Br J Cancer. 1995;72:41–44. doi: 10.1038/bjc.1995.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dueñas-Gonzalez A, Isales CM, Abad-Hernandez MM, Gonzalez-Sarmiento R, Sangueza O, Rodriguez-Commes J. Expression of inducible nitric oxide synthase in breast cancer correlates with metastatic disease. Mod Pathol. 1997;10:645–9. [PubMed] [Google Scholar]
- 109.Fujimoto H, Ando Y, Yamashita T, et al. Nitric oxide synthase activity in human lung cancer. Jpn J Cancer Res. 1997;88:1190–8. doi: 10.1111/j.1349-7006.1997.tb00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ambs S, Merriam WG, Bennett WP, et al. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334–41. [PubMed] [Google Scholar]
- 111.Lewis NP, Tsao OPS, Rickenbacher PR, et al. Induction of nitric oxide synthase in the human cardiac allograft is associated with contractile dysfunction of the left ventricle. Circulation. 1996;93:720–9. doi: 10.1161/01.cir.93.4.720. [DOI] [PubMed] [Google Scholar]
- 112.McDermott CD, Gavita SM, Shennib H, Giaid A. Immunohistochemical localization of nitric oxide synthase and the oxidant peroxynitrite in lung transplant recipients with obliterative bronchiolitis. Transplantation. 1997;64:270–4. doi: 10.1097/00007890-199707270-00016. [DOI] [PubMed] [Google Scholar]
- 113.Moilanen E, Moilanen T, Knowles R, Charles I, Kadoya Y, Al-Saffar N, Revell PA, Moncada S. Nitric oxide synthase is expressed in human macrophages during foreign body inflammation. Am J Pathol. 1997;150:881–7. [PMC free article] [PubMed] [Google Scholar]
- 114.Watkins SC, Macaulay W, Turner D, Kang R, Rubash HE, Evans CH. Identification of inducible nitric oxide synthase in human macrophages surrounding loosened hip prostheses. Am J Pathol. 1997;150:1199–206. [PMC free article] [PubMed] [Google Scholar]
- 115.Hukkanen M, Corbett SA, Batten J, et al. Aseptic loosening of total hip replacement. Macrophage expression of inducible nitric oxide synthase and cyclo-oxygenase-2 together with peroxynitrite formation, as a possible mechanism for early prosthesis failure. J Bone Joint Surg Br. 1997;79-B:467–74. doi: 10.1302/0301-620x.79b3.7469. [DOI] [PubMed] [Google Scholar]
- 116.Yang CC, Alvarez RB, Engel WK, Askanas V. Increase of nitric oxide synthases and nitrotyrosine in inclusion-body myositis. Neuroreport. 1996;8:153–8. doi: 10.1097/00001756-199612200-00031. [DOI] [PubMed] [Google Scholar]
- 117.Wildhirt SM, Suzuki S, Wolf WP, Dudek R, Horstman D, Weismueller S, Reichart B. S-Methylisothiourea inhibits inducible nitric oxide synthase and improves left ventricular performance after acute myocardial infarction. Biochem Biophys Res Commun. 1996;227:328–33. doi: 10.1006/bbrc.1996.1509. [DOI] [PubMed] [Google Scholar]
- 118.Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20:132–9. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- 119.Lejeune P, Lagadec P, Onier N, Pinard D, Ohshima H, Jeannin JF. Nitric oxide involvement in tumor-induced immunosuppression. J Immunol. 1994;152:5077–83. [PubMed] [Google Scholar]
- 120.Jenkins DC, Charles IG, Thomsen LL, et al. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci USA. 1995;92:4392–6. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Suschek C, Schuy V, Bruch-Gerhartz D, Kröncke KD, Krutmann J, Kolb-Bachofen V. Nitric oxide protects endothelial cells from UVA-induced apoptosis. Immunobiol. 1997;197:297. [Google Scholar]
- 122.Tzeng E, Billiar RB. Nitric oxide and the surgical patient. Identifying therapeutic targets. Arch Surg. 1997;132:977–82. doi: 10.1001/archsurg.1997.01430330043006. [DOI] [PubMed] [Google Scholar]
- 123.Clark RSB, Kochanek PM, Schwarz MA, Schiding JK, Turner DS, Chen M, Carlos TM, Watkins SC. Inducible nitric oxide synthase expression in cerebrovascular smooth muscle and neutrophils after traumatic brain injury in immature rats. Pediatr Res. 1996;39:784. doi: 10.1203/00006450-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 124.Nussler AK, Di Silvio M, Billiar TR, Hoffman RA, Geller DA, Selby R, Madariaga J, Simmons RL. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992;176:261–4. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suschek C, Bruch D, Kröncke KD, Kolb-Bachofen V. Inducible nitric oxide synthase in human dermal endothelial cells. Eur J Clin Invest. 1996;26(Suppl. 1):A4. [Google Scholar]
- 126.Takeichi O, Saito I, Okamoto Y, Tsurumachi T, Saito T. Production of human-inducible nitric oxide synthase in radicular cysts. J Endodont. 1998;24:157–60. doi: 10.1016/S0099-2399(98)80173-8. [DOI] [PubMed] [Google Scholar]
- 127.Eizirik DL, Pipeleers DG, Ling Z, Welsh N, Hellerström C, Andersson A. Major species differences between humans and rodents in the susceptibility to pancreatic β-cell injury. Proc Natl Acad Sci USA. 1994;91:9253–6. doi: 10.1073/pnas.91.20.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bellmann K, Jäättelä M, Wissing D, Burkart V, Kolb H. Heat shock protein hsp70 overexpression confers resistance against nitric oxide. FEBS Letters. 1996;391:185–8. doi: 10.1016/0014-5793(96)00730-2. [DOI] [PubMed] [Google Scholar]
- 129.Bellmann K, Liu H, Burkart V, Pozzilli P, Cavollo MG, Jäättelä M, Steensgaard P, Kolb H. Immunobiol. 1997;197:230. [Google Scholar]
- 130.Berendji D, Kröncke KD, Kolb-Bachofen V. Reversible inactivation of the zinc finger transcription factor Sp 1 by nitric oxide suppresses IL-1β-mediated IL-2 gene expression in lymphocytes. Immunobiol. 1997;197:182–3. [Google Scholar]
- 131.Dimmeler S, Haendeler J, Nehls M, Zeiher AM. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1β-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J Exp Med. 1997;185:601. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Melino G, Bernassola F, Knight RA, Corasaniti MT, Nisticò G, Finazzi-Agrò A. S-nitrosylation regulates apoptosis. Nature. 1997;388:432. doi: 10.1038/41237. [DOI] [PubMed] [Google Scholar]