Abstract
A reduced prevalence of pigeon fanciers' lung has been reported in pigeon breeders who smoke cigarettes. Serum and salivary antibodies to pigeon intestinal mucin and pigeon serum proteins were investigated in 227 pigeon fanciers, subdivided according to smoking habit and clinical status. Smokers had a lower incidence of precipitating antibodies to pigeon antigens and lower titres of serum IgG and IgA antibodies to mucin and to pigeon serum proteins in ELISA compared with non-smokers and ex-smokers. In contrast, IgG antibody titres to tetanus toxoid were similar in smoking and non-smoking groups. In contrast to serum antibodies, salivary IgA antibody titres to pigeon antigens were similar in smokers and non- or ex-smokers. Approximately one third of the smokers reported symptoms consistent with pigeon fanciers' lung but did not have precipitating antibodies. Only some individuals with precipitating antibodies had disease symptoms, and IgG antibody titres in these individuals were not significantly higher than in many asymptomatic individuals. Salivary IgA titres against pigeon mucin were significantly higher in asymptomatic individuals, consistent with a protective role for these antibodies. The results confirm that smoking is associated with a decreased serum antibody response to inhaled pigeon antigens, affecting IgG1, IgG2 and IgA responses, but this impairment does not extend to salivary IgA or to antibody responses to a parenterally administered protein antigen. The fact that responses to pigeon serum proteins and to pigeon intestinal mucin were similarly affected suggests that cigarette smoking depresses both T-independent and T-dependent responses to inhaled antigens.
Keywords: pigeon fanciers' lung, extrinsic allergic alveolitis, smoking, antibody response, salivary IgA
INTRODUCTION
Pigeon fanciers' lung (PFL) is a form of extrinsic allergic alveolitis (EAA) caused by hypersensitivity reactions to inhaled pigeon antigens in the lung of a sensitized host. The disease is associated with both respiratory and systemic symptoms, occurring some hours after exposure to antigen. The delay between exposure and the onset of symptoms, together with the presence of antibodies to pigeon antigens in symptomatic pigeon breeders [1–5], suggests a role for immune complex (type III) reactions. The finding of large numbers of infiltrating T lymphocytes in the lesions and granuloma formation suggests cell-mediated hypersensitivity may also be important [6].
There is a large variation in both the clinical and immune responses of individuals exposed to the antigens associated with PFL [7,8], and it is unclear why only a proportion of exposed individuals develop the disease. Cigarette smokers show a reduced prevalence of PFL [9,10], and this is also true for other forms of EAA [11–14]. This appears to be related to reduced humoral immune responses: smokers produce lower concentrations of specific serum and bronchial lavage fluid IgG to inhaled pigeon antigens [15–17] and are less likely to produce antibodies associated with farmers' lung, another form of EAA [18].
Antibodies against a range of pigeon antigens can be demonstrated in both symptomatic and healthy exposed individuals. In recent years attention has focused on IgA as the major antigenic component in pigeon secretory materials. More recently we have identified antibodies to another, unrelated antigen, pigeon intestinal mucin [19,20]. Mucin is abundantly present in extracts of pigeon droppings and bloom [21], and the presence of high IgG1 titres to this antigen correlates with the development of disease [8].
In this study we investigate the antibody responses to pigeon mucin and pigeon serum proteins in a large group of clinically characterized pigeon breeders. Serum IgG, IgG subclasses and IgA are compared between smokers, ex-smokers and non-smokers.
Salivary IgA antibodies are also measured, to elucidate further the pattern of humoral immune responses in these groups.
SUBJECTS AND METHODS
Subjects and clinical status
During two conventions of pigeon breeders, in Peterlee and Blackpool (UK) in 1995/1996, and two visits to marking stations in 1995, a self-administered questionnaire was completed by 227 individuals. Clinical information relating to the nature, frequency and severity of late (4–8 h) respiratory and systemic symptoms related to pigeon contact was obtained as well as further relevant background details such as degree of pigeon contact, smoking history and other respiratory symptoms in accordance with the Medical Research Council (MRC) questionnaire on chronic bronchitis. A clinical diagnosis of symptoms related to PFL was based on the presence of at least one classic respiratory symptom, such as shortness of breath or persistent dry cough, and at least one classic systemic symptom, such as fever with shivering or aching muscles, occurring on at least three separate occasions 4–8 h after pigeon contact.
Each individual donated a blood sample from which the plasma was taken to study various immunological parameters. Samples of saliva were also collected from each person. All samples were stored as aliquots at −80°C.
Precipitating antibodies to pigeon faeces and pigeon serum were detected by counter current immunoelectrophoresis (CIE) [22] using antigen and positive control sera obtained from Microgen Ltd (Penarth, UK). Only those sera that demonstrated precipitins to both pigeon faeces and pigeon serum were considered to be precipitating antibody-positive.
With the clinical and precipitating antibody information each individual was classified as being in one of four groups depending on the presence or absence of symptoms and the presence or absence of precipitating antibody:
Group A, symptomatic with precipitating antibodies
Group B, asymptomatic with precipitating antibodies
Group C, symptomatic without precipitating antibodies
Group D, asymptomatic without precipitating antibodies
Only those individuals in Group A were considered to have classic PFL.
Current and ex-smokers were asked how long they had been smoking and to estimate the average number of cigarettes smoked per day. The number of pack years smoked was calculated from this (pack/years = average daily consumption × number of years smoking/20) and this was used as a measure of exposure to cigarette smoke. The participants in this study were volunteers, and previous studies measuring the end expired carbon monoxide as a marker of smoking levels of pigeon fanciers suggest that the smoking histories given by this group are accurate [23].
Antigens
Three racing pigeons were exsanguinated. Sera were collected and stored as aliquots at −80°C. Tetanus vaccine BP in simple solution (Tet/Vac/Ft; The Wellcome Foundation, Beckenham, UK) was used as antigen when measuring anti-tetanus immune responses. One millilitre vaccine contained 28 Lf toxoid of Clostridium tetani exotoxin. Mucin was prepared from the intestines of freshly killed pigeons, as previously described [8].
Assays for total immunoglobulins
Total plasma IgG, IgA and IgM (mg/ml) were quantified by automated rate nephelometry using a Beckman (High Wycombe, UK) array protein system. Total IgE (U/ml) was measured by ELISA, as previously described [8].
Assays for specific antibodies
IgG and IgG subclass antibodies to pigeon serum and pigeon intestinal mucin were quantified by ELISA, as previously described [8]. IgA (serum and salivary) antibodies were measured using an assay identical to the IgG ELISA except for the antibody conjugate, which was horseradish peroxidase (HRP)–goat anti-human IgA (Sigma Immuno-Chemicals, Poole, UK). Secretory antibodies were measured using the same assay but with a secretory component-specific conjugate (HRP–sheep anti-human secretory component; The Binding Site, Birmingham, UK).
The assay for IgG anti-tetanus toxoid was essentially the same as that described for IgG antibodies to pigeon serum, with the following minor modifications. Plates were coated overnight at 4°C with tetanus vaccine (1/100 in borate-buffered saline, pH 8.5). After washing in PBS–Tween (PBS–T) the plates were blocked with PBS–T containing 0.5% bovine serum albumin (BSA) for 45 min. The plates were washed three times in PBS–T and then the samples were added, as previously, diluted in PBS–T–0.5% BSA. The plates were incubated for 2 h at 37°C, washed three times in PBS–T and then incubated with HRP-conjugated rabbit anti-human IgG for 1 h at 37°C.
For all assays absorbance was plotted against serum dilution, and the titre was calculated for each serum as the reciprocal of the dilution giving an optical density (OD) of 0.2.
Statistical analysis
Statistical analysis was performed using Student's t-test where the data were normally distributed and the Mann–Whitney U-test where the data were non-parametric. The significance of contingency tables was assessed either by χ2 analysis or Fisher's exact test. All statistical calculations were carried out using a computer-based, commercially available statistics package (Minitab data analysis software). P < 0.05 was considered significant.
RESULTS
Smoking and clinical status
All 227 individuals reported in this communication were male. Of these, 56 smoked cigarettes, 70 were ex-smokers and 101 had never smoked. Within each group details of age, degree of pigeon exposure, and clinical status are given in Table 1.
Table 1.
Background details of pigeon fanciers in study
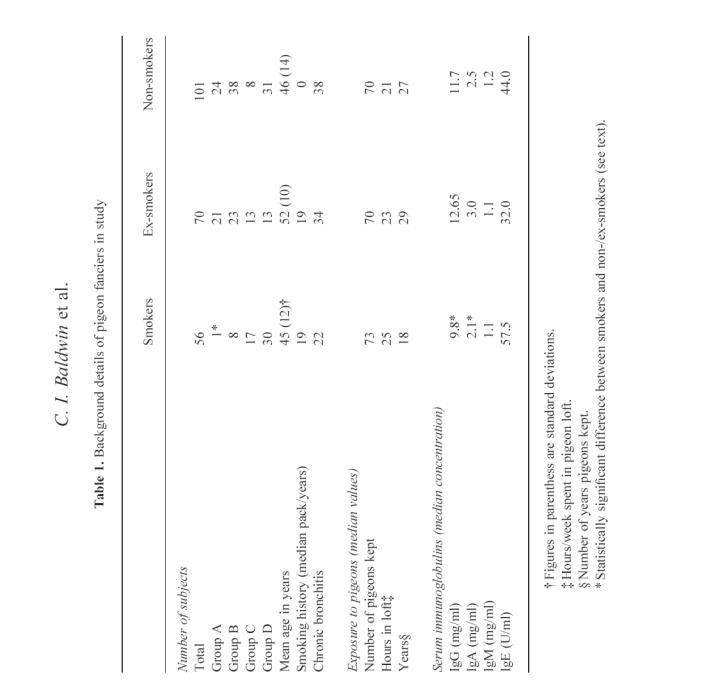
The mean age of all three groups was similar, although smokers were significantly younger than ex-smokers but not non-smokers. All three groups were similar in terms of their level of exposure to pigeons, and both smokers and ex-smokers were similar in terms of pack/years of cigarettes smoked.
Of the 227 individuals, 115 had precipitating antibodies to pigeon antigens. Of these 46 were in Group A, fulfilling our criteria for the diagnosis of PFL, and 69 had precipitating antibody with no apparent symptoms (Group B). Of the 112 individuals who did not have precipitating antibodies, 38 reported avian-related symptoms (Group C) and 74 were asymptomatic (Group D). Smokers were significantly less likely to have precipitating antibody than ex-smokers or non-smokers (Fisher's exact test P = 0.0001 and P = 0.0009, respectively), whilst there was no significant difference between ex-smokers or non-smokers. The incidence of PFL (Group A) was significantly higher in the non-smoking and ex-smoker groups (between non-smokers and current smokers χ2 = 14.647, P < 0.01; between ex-smokers and current smokers χ2 = 16.507, P < 0.01), whilst there was no difference in prevalence of disease between the non-smokers and ex-smokers (χ2 = 1.754, P > 0.1).
Chronic bronchitis has been suggested to form part of the clinical spectrum in PFL. In this study 94 individuals had symptoms in keeping with chronic bronchitis, and there was no significant difference between smoking and non-smoking groups. This probably reflects the conflicting influences of PFL and cigarette smoking, since the latter is the major risk factor for development of chronic bronchitis in the general population.
Smoking and total serum immunoglobulin
The median total serum IgG, IgA, IgM and IgE levels, within each group, are shown in Table 1. Smokers had significantly less IgG and IgA than non-smokers and ex-smokers (P < 0.01 in all cases). There were no significant differences between groups for serum IgM or IgE, and no difference between ex-smokers and non-smokers for IgG and IgA.
Smoking and specific antibodies
Serum antibody titres to pigeon mucin and pigeon serum were measured in each individual (Fig. 1 and Tables 2 and 3). Smokers had much lower titres of IgG against both antigens than either non-smokers or ex-smokers (P < 0.02). Ex-smokers had somewhat higher titres of IgG against both antigens than non-smokers, but this difference was only significant for pigeon serum.
Fig. 1.
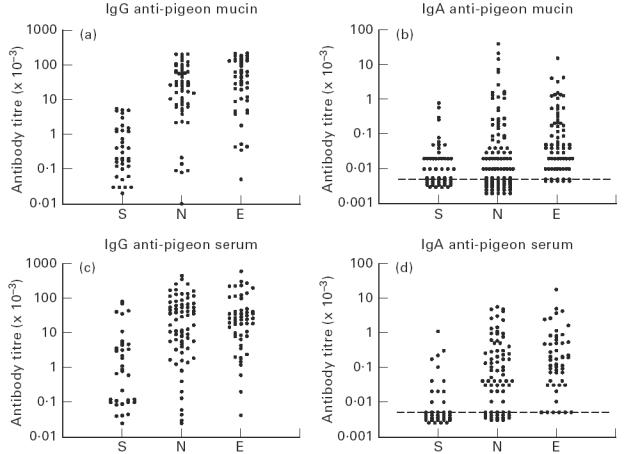
Serum antibody titres of pigeon fanciers against pigeon mucin and pigeon serum: S, Smokers; N, non-smokers; E, ex-smokers. Each point represents one individual.
Table 2.
Effect of smoking on IgG and IgA antibodies†
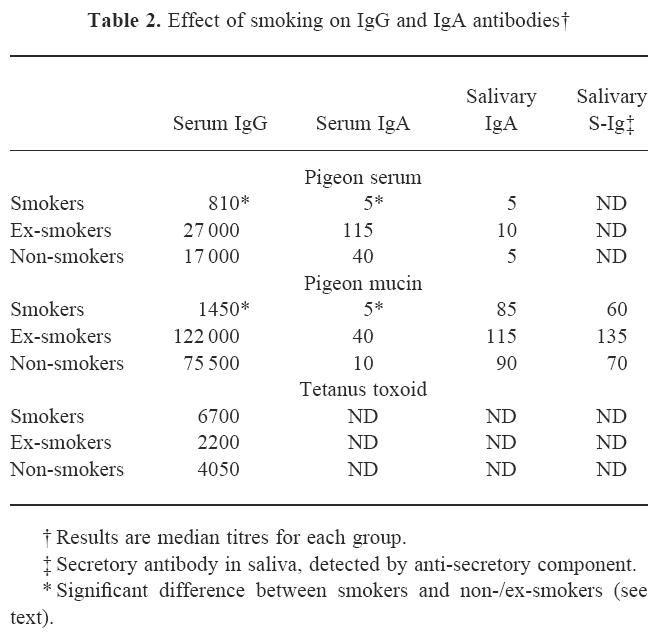
Table 3.
Effect of smoking on IgG subclass antibodies†
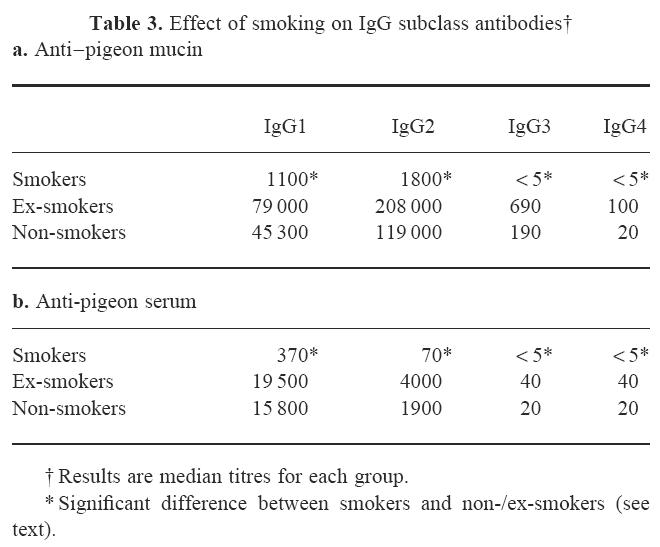
The IgG subclass titres are shown in Table 3. Subclass profiles differed between the two antigens, in that the anti-pigeon serum antibodies were dominated by IgG1, whereas antibodies to mucin also had a major IgG2 component. All subclasses were found to be reduced in smokers compared with ex- and non-smokers (Table 3). This difference was significant for both antigens and all four subclasses. No significant differences were seen between ex-smokers and non-smokers, except in IgG4, where ex-smokers had significantly higher titres against both antigens. However, IgG4 titres were extremely low in all groups.
Serum IgA antibody titres to both antigens were 2–4 orders lower than IgG titres, but showed a similar pattern in being significantly lower in smokers than in non- or ex-smokers (P < 0.02) (Fig. 1 and Table 2). Again, titres were somewhat higher in ex-smokers than in non-smokers, and this difference was significant for both antigens (P < 0.05).
In contrast to the serum antibody titres, salivary IgA antibodies against pigeon mucin were not significantly reduced in smokers compared with ex- or non-smokers (Table 2). Salivary IgA antibodies against pigeon serum tended to be very low (undetectable in most individuals), and no significant differences were seen between the groups. To confirm that the salivary anti-mucin IgA antibodies did indeed reflect a mucosal immune response the assays were repeated using anti-secretory component instead of anti-IgA: the titres were very similar and, again, smokers were not significantly different from non- and ex-smokers.
For comparison, IgG antibodies to tetanus toxoid, a parenterally administered protein antigen, were also measured. Smokers showed no impairment of response to this antigen, in fact titres were somewhat higher than in non-smokers and ex-smokers (Table 2).
Specific antibodies and clinical status
A comparison of the IgG and IgA antibody titres to pigeon serum and pigeon mucin in sera from each clinical group is shown in Fig. 2. Serum IgG and IgA antibodies showed a similar pattern: titres were much higher in people with precipitating antibodies, whether symptomatic (Group A) or asymptomatic (Group B). Salivary IgA antibodies to pigeon intestinal mucin showed a completely different pattern: titres were significantly higher in asymptomatic (Groups B and D) than in symptomatic individuals (Groups A and C: P < 0.01). All four groups showed similar, very low or undetectable levels of salivary IgA against pigeon serum.
Fig. 2.
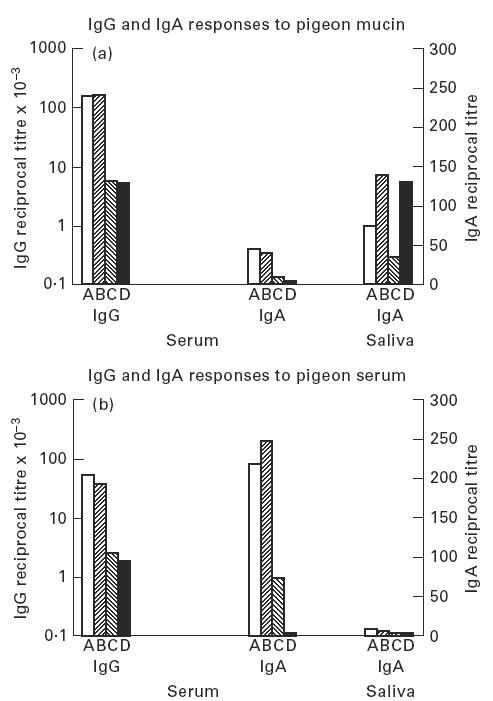
Antibody titres to (a) pigeon mucin and (b) pigeon serum in pigeon fanciers subdivided by clinical group. Median values shown for each group. Group A precipitin+/symptomatic, Group B precipitin+/asymptomatic, Group C precipitin−/symptomatic, Group D precipitin−/asymptomatic.
DISCUSSION
This study demonstrates that pigeon fanciers who smoke show depressed antibody responses to pigeon antigens compared with non-smokers. Both the incidence of precipitating antibodies, as measured by CIE, and the antibody titre in ELISA to both pigeon serum proteins and the recently described novel antigen, pigeon intestinal mucin, were reduced in smokers. The effect was not isotype-specific, since both IgG and IgA titres were significantly lower in smokers, and all four IgG subclasses were affected. In contrast to the systemic antibody response, local salivary IgA responses were not depressed in smokers.
A decreased incidence of disease associated with reduced antibody responses in smokers has been observed in EAA caused by Trichosporon cutaneum [14] and farmers' lung [13,18], but the mechanism is unclear. Effects of smoking on the airways might result in altered exposure of the mucosal associated lymphoid tissues to pigeon antigens. Alternatively or additionally, smoking may directly affect cells involved in the production of an antibody response. Exposure to cigarette smoke has been reported to depress antibody responses to sheep erythrocytes administered intratracheally in mice [24]. The mechanisms involved in this phenomenon are unclear. Direct effects on lymphocytes might be involved, and nicotine has been shown to impair antigen receptor-mediated signal transduction in rat lymphocytes [25]. Effects on antigen-presenting or other accessory cells might also be important. Alveolar macrophages from smokers, compared with non-smokers, show decreased accessory cell function in mitogen responses, express fewer HLA-DR antigens and show decreased production of certain cytokines including IL-1 and IL-6 [26–29].
Antibody titres against tetanus toxoid, a parenterally administered antigen, were not decreased in smokers compared with non-smokers, suggesting that the effects of smoking mainly operate on responses to inhaled antigens. However, in other studies a negative influence of cigarette smoking was reported in subjects immunized intramuscularly with hepatitis B vaccine [30,31]. In our study the total serum levels of IgG and IgA were lower in the smokers, possibly reflecting a general suppression of humoral immunity.
In contrast to the negative effects on IgG antibody responses observed in this and other studies, cigarette smoking has been associated with increased serum IgE levels [32–34]. Smokers tend to produce IgE antibodies in response to inhaled prawn antigens in seafood factory workers [35] and in response to inhaled antigens from a contaminated humidifier [36], whilst IgG was the predominant isotype in non-smokers. Moreover, peripheral blood lymphocytes from smokers tend to secrete greater quantities of the Th2 cytokine IL-4, which is known to be important in regulating IgE production [37]. Thus the effect of smoking, rather than simply being immunosuppressive, may be to modulate the qualitative nature of an immune response and favour Th2 activation. Th2 cytokines have been implicated not only in IgE production, but also in the production of IgA and IgG4. However, in this study serum IgA antibodies against pigeon antigens were depressed in smokers, and IgG4, although a minor subclass, was similarly affected. It will, however, be of interest to investigate T cell responses to pigeon antigens in these groups to determine whether differences in cytokine production might be related to the decreased antibody production in smokers.
Antibodies to pigeon mucin and to pigeon serum proteins differ with respect to their isotype composition [8]. The high IgG2 titres against serum mucin suggest that this response is at least partly T-independent, consistent with the highly glycosylated nature of the molecule. In contrast, the high IgG1 titres against pigeon serum proteins are more typical of a T-dependent antigen. It seems likely therefore that whatever the mechanism by which smoking down-regulates antibody production to inhaled antigens, T-independent and T-dependent responses are similarly affected. Also of note were the higher serum IgA antibody titres against pigeon serum proteins compared with mucin: interestingly, however, this pattern was reversed in the salivary IgA antibodies where titres against mucin were much higher than those against pigeon serum proteins. In another study salivary IgA antibodies to pigeon serum proteins were observed in patients with PFL, but also in healthy, unexposed control subjects [38]. In contrast, salivary IgG values were increased in subjects with PFL relative to controls. This finding accords with our own unpublished observations that serum and salivary IgG antibody titres show a strong positive correlation, and is consistent with the salivary IgG reflecting the systemic immune response, perhaps being passively exuded from serum.
In contrast to the serum IgA antibodies, titres of salivary IgA against pigeon antigens showed no significant difference between smokers and non-smokers. This confirms the independence of the mucosal and systemic IgA responses to these antigens.
The finding that serum antibody titres are similar in ex- and non-smokers indicates that the suppressive effect of smoking is reversible. This may be of clinical relevance for pigeon fanciers who stop smoking: these individuals are consequently likely to develop high levels of antibodies against pigeon antigens, and should perhaps be advised to take particular care to avoid sensitization.
Diagnosis of PFL can be problematic. In this study we relied upon the presence of both local and systemic reactions, occurring 4–8 h after exposure, together with precipitating antibodies to pigeon antigens. It was of note, however, that half of the individuals in this study who reported pigeon-related symptoms did not have precipitating antibodies to pigeon antigens and had only low titres of antibody in ELISA (Group C). Moreover, one third of the smokers fell into this category, whilst only 17/125 (≈ 1 in 7) non- or ex-smokers were in this group. Because smokers may experience respiratory problems as a direct result of their smoking, it would be of interest to investigate whether these individuals respond to avian antigens after inhalation challenge. It is possible that this group may represent a form of PFL less dependent on large quantities of serum antibody, and studies of T cell responses to pigeon antigens in these individuals may further clarify the situation. Interestingly, when individuals were grouped purely on the basis of whether or not they had avian-related symptoms, asymptomatic pigeon fanciers had significantly higher titres of salivary IgA than symptomatics. The significance of this finding is not clear, but would be consistent with a protective effect of a local immune response against systemic sensitization.
Acknowledgments
The authors would like to thank Dr G. Boyd and Dr P. Lynch for inviting and helping us to collect samples at the British Pigeon Fanciers Medical Research stall at both Peterlee and Blackpool conventions. We are grateful to Ms Beverley Stevens for expert technical assistance. This study was supported by a grant from the Wellcome Trust, grant reference number 042462.
REFERENCES
- 1.Barboriak JJ, Sosman AJ, Reed CE. Serological studies in pigeon breeders' disease. J Lab Clin Med. 1965;65:600–4. [PubMed] [Google Scholar]
- 2.Fink JN, Tebo T, Barboriak JJ. Differences in immune responses of pigeon breeders to pigeon serum proteins. J Lab Clin Med. 1969;74:325–30. [PubMed] [Google Scholar]
- 3.Hargreave FE, Pepys J. Allergic respiratory reactions in bird fanciers provoked by allergen inhalation provocation tests. J Allergy Clin Immunol. 1972;50:157–73. doi: 10.1016/0091-6749(72)90047-4. [DOI] [PubMed] [Google Scholar]
- 4.Tebo HT, Fredricks WW, Roberts RC. The antigens of pigeon breeders disease. II. Isolation and characterisation of antigen PDE I. Int Arch Allergy Appl Immunol. 1977;54:553–9. doi: 10.1159/000231876. [DOI] [PubMed] [Google Scholar]
- 5.Banham SW, MacKenzie H, McSharry C, Lynch PP, Boyd G. Antibody against a pigeon bloom extract: a further antigen in pigeon fanciers' lung. Clin Allergy. 1982;12:173–8. doi: 10.1111/j.1365-2222.1982.tb01636.x. [DOI] [PubMed] [Google Scholar]
- 6.Hensley GT, Garancis JC, Cherayil GD, Fink JN. Lung biopsies of pigeon breeders' disease. Arch Pathol. 1969;87:572–9. [PubMed] [Google Scholar]
- 7.Bourke S, Boyd G. Pigeon fanciers' lung. Br Med J. 1997;315:70–71. doi: 10.1136/bmj.315.7100.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldwin CI, Todd A, Bourke SJ, Allen A, Calvert JE. IgG subclass responses to pigeon intestinal mucin are related to development of pigeon fanciers' lung. Clin Exp Allergy. 1988;28:349–57. doi: 10.1046/j.1365-2222.1998.00238.x. [DOI] [PubMed] [Google Scholar]
- 9.Boyd G, Madkour M, Middleton S, Lynch PP. Effect of smoking on circulating antibody levels to avian protein in pigeon breeders disease. Thorax. 1977;32:651. [Google Scholar]
- 10.Warren CPW. Extrinsic allergic alveolitis: a disease commoner in non-smokers. Thorax. 1977;32:567–9. doi: 10.1136/thx.32.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan D, Schmyth JT, Lester RC, Pethysbridge R. Chest symptoms and farmers' lung: a community survey. Br J Ind Med. 1973;30:259–61. doi: 10.1136/oem.30.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cormier Y, Belanger J, Durand P. Factors affecting the development of serum precipitins to farmers lung antigen in Quebec dairy farmers. Thorax. 1985;40:138–42. doi: 10.1136/thx.40.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terho EO, Husman K, Vohlonen I. Prevalence and incidence of chronic bronchitis and farmers lung with respect to age, sex, atopy and smoking. Eur J Resp Dis. 1987;71(Suppl. 152):19–28. [PubMed] [Google Scholar]
- 14.Arima K, Ando M, Ito K, Sakata T, Yamaguchi T, Araki S, Futatsuka M. Effect of cigarette smoking on prevalence of summer-type hypersensitivity pneumonitis caused by Trichosporon cutaneum. Arch Environ Health. 1992;47:274–8. doi: 10.1080/00039896.1992.9938361. [DOI] [PubMed] [Google Scholar]
- 15.McSharry C, Banham SW, Boyd G. The effect of cigarette smoking on the antibody response to inhaled antigens and the prevalence of extrinsic allergic alveolitis among pigeon breeders. Clin Allergy. 1985;15:487–94. doi: 10.1111/j.1365-2222.1985.tb02299.x. [DOI] [PubMed] [Google Scholar]
- 16.Carrilli T, De Castro FR, Cuevas M, Diaz F, Cabrera P. Effect of cigarette smoking on the humoral immune response in pigeon fanciers. Allergy. 1991;46:241–4. doi: 10.1111/j.1398-9995.1991.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds SP, Edwards JH, Jones KP, Davies BH. Immunoglobulin and antibody levels in bronchoalveolar lavage fluid from symptomatic and asymptomatic pigeon breeders. Clin Exp Immunol. 1991;86:278–85. doi: 10.1111/j.1365-2249.1991.tb05810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tehro EO, Husman K, Vohlonen I, Mantyjarvi RA. Serum precipitins against microbes in mouldy hay with respect to age, sex, atopy, and smoking of farmers. Eur J Resp Dis. 1987;71(Suppl. 152):115–21. [PubMed] [Google Scholar]
- 19.Todd A, Coan RM, Allen A. Pigeon breeders' lung: pigeon intestinal mucin, an antigen distinct from pigeon IgA. Clin Exp Immunol. 1991;85:453–8. doi: 10.1111/j.1365-2249.1991.tb05748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todd A, Coan RM, Allen A. Pigeon breeders' lung: IgG subclasses to pigeon intestinal mucin and IgA antigens. Clin Exp Immunol. 1993;92:494–9. doi: 10.1111/j.1365-2249.1993.tb03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldwin CI, Stevens B, Connors S, Todd A, Bourke SJ, Calvert JE, Allen A. Pigeon fanciers' lung: the mucin antigen is present in pigeon droppings and pigeon bloom. Int Arch Allergy Appl Immunol. 1998 doi: 10.1159/000024009. manuscript submitted. [DOI] [PubMed] [Google Scholar]
- 22.Mackenzie DWR. Public Health Laboratory Service Monograph Series. London: HMSO; 1980. Basic serodiagnostic methods for diseases caused by fungi and actinomyces. [Google Scholar]
- 23.Anderson K, Morrison SM, Bourke S, Boyd G. Effect of cigarette smoking on the specific antibody response in pigeon fanciers. Thorax. 1988;43:798–800. doi: 10.1136/thx.43.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas W, Holt PG, Keast D. Effect of cigarette smoke on primary and secondary humoral responses of mice. Nature. 1973;243:240–1. doi: 10.1038/243240a0. [DOI] [PubMed] [Google Scholar]
- 25.Geng Y, Savage SM, Johnson LJ, Seagrave J, Sopori ML. Effects of nicotine on the immune response. I. Chronic exposure to nicotine impairs antigen receptor-mediated signal transduction in lymphocytes. Toxicol Appl Pharmacol. 1995;135:268–78. doi: 10.1006/taap.1995.1233. [DOI] [PubMed] [Google Scholar]
- 26.McCrea KA, Ensor JE, Nall K, Bleecker ER, Hadsay JD. Altered cytokine regulation in the lungs of cigarette smokers. Am J Resp Crit Care Med. 1994;150:696–703. doi: 10.1164/ajrccm.150.3.8087340. [DOI] [PubMed] [Google Scholar]
- 27.Sauty A, Mauel J, Philippeaux MM, Leuenberger P. Cytostatic activity of alveolar macrophages from smokers and non-smokers: role of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha. Am J Resp Cell Mol Biol. 1994;11:631–7. doi: 10.1165/ajrcmb.11.5.7946392. [DOI] [PubMed] [Google Scholar]
- 28.Twigg HL. Impaired alveolar macrophage accessory cell function and reduced incidence of lymphocytic alveolitis in HIV-infected patients who smoke. AIDS. 1994;8:611–8. doi: 10.1097/00002030-199405000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Pankow W, Neumann K, Ruschoff J, von Wichart P. Human alveolar macrophages; comparison of cell size, autofluorescence, and HLA-DR antigen expression in smokers and non-smokers. Cancer Detect Prev. 1995;19:268–73. [PubMed] [Google Scholar]
- 30.Struve J, Aronsson B, Frenning B, Granath F, von Sydow M, Weiland O. Intramuscular versus intradermal administration of a recombinant hepatitis B vaccine: a comparison of response rates and analysis of factors influencing the antibody response. Scand J Infect Dis. 1992;24:423–9. doi: 10.3109/00365549209052627. [DOI] [PubMed] [Google Scholar]
- 31.Roome AJ, Walsh SJ, Cartter ML, Hadler JL. Hepatitis B vaccine responsiveness in Connecticut public safety personnel. JAMA. 1993;270:2931–4. [PubMed] [Google Scholar]
- 32.Warren CPW, Holford Stevens V, Wong C, Manfreda J. The relationship between smoking and total immunoglobulin E levels. J Allergy Clin Immunol. 1982;69:370–5. doi: 10.1016/0091-6749(82)90148-8. [DOI] [PubMed] [Google Scholar]
- 33.Bahna SL, Heiner DC, Myhre BA. Immunoglobulin E pattern in cigarette smokers. Allergy. 1983;38:57–64. doi: 10.1111/j.1398-9995.1983.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 34.Criqui M, Seibles J, Hamburger R, Coughlin S, Gabriel S. Epidemiology of immunoglobulin E levels in a defined population. Ann Allergy. 1990;64:308–13. [PubMed] [Google Scholar]
- 35.McSharry C, Anderson K, McKay IC, Colloff MJ, Feyerabend C, Wilson RB, Wilkinson PC. The IgE and IgG antibody responses to aerosols of Nephrops norvegicus (prawn) antigens: the association with clinical hypersensitivity and with cigarette smoking. Clin Exp Immunol. 1994;97:499–504. doi: 10.1111/j.1365-2249.1994.tb06116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finnegan MJ, Little S, Gordon DJ, Austwick PKC, Tee RD, Nunn AJ, Newman-Taylor AJ. The effect of smoking on the development of allergic disease and specific immunological responses in a factory workforce exposed to humidifier contaminants. Br J Ind Med. 1991;48:30–33. doi: 10.1136/oem.48.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byron KA, Varigos GA, Wootton AM. IL-4 production is increased in cigarette smokers. Clin Exp Immunol. 1994;95:333–6. doi: 10.1111/j.1365-2249.1994.tb06533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendoza F, Baltazares M, Ramirez A, Sansores R, Nava A, Banales JL, Selman M. Detection of salivary and seric IgG and IgA antipooled pigeon sera activities in patients with pigeon breeders' disease. J Clin Lab Anal. 1996;10:149–54. doi: 10.1002/(SICI)1098-2825(1996)10:3<149::AID-JCLA7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]


