Abstract
Expression of HLA class II molecules on thyrocytes is a characteristic feature of autoimmune thyroid disease and may lead the thyroid cells to present autoantigens to CD4+ T lymphocytes. Since HLA-DM is a critical molecule in class II-restricted antigen processing and presentation, we assessed the expression of HLA-DMB, -invariant chain (Ii), class II transactivator (CIITA) and DRA in an untransformed, pure thyrocyte strain HTV-59A. Here we report that both HLA-DMB mRNA and the protein are expressed in thyrocytes and that CIITA expression is enhanced by interferon-gamma (IFN-γ) treatment and occurs before DMB, Ii and DRA up-regulation, suggesting CIITA expression is a requirement for antigen processing in thyrocytes. These results indicate that thyrocytes are capable of antigen processing and possibly antigen presentation to T cells.
Keywords: HLA-DMB, Ii, CIITA, thyrocytes
INTRODUCTION
The thyroid gland is the target of Hashimoto's thyroiditis and Graves' disease (GD): autoimmune thyroid diseases (AITD) with opposite clinical outcomes. The pathogenesis of Hashimoto's thyroiditis is primarily due to T cell-mediated immune destruction of the thyroid and the typical histology includes lymphocytic infiltration of the gland. These features are also found, but to a highly variable degree, in thyroids of patients with GD, in which the hyperthyroidism is induced by a thyroid-stimulating antibody [1]. Although the two disorders have striking differences in clinical symptoms, they share the same histopathological features: lymphocytic infiltration and aberrant expression of HLA class II molecules on thyrocytes [2].
HLA class II molecules are heterodimeric, polymorphic transmembrane glycoproteins expressed on cells of the immune system and on the affected cells of autoimmunity [3]. For recognition of antigen by CD4+ T cells, proteins must undergo partial degradation into peptides and the resulting peptides must be bound to MHC class II molecules and carried with them to the cell surface [4].
Two novel genes, HLA-DMA and DMB, have recently been identified and latterly demonstrated to be important for antigen processing and presentation [5–7]. Their function is to catalyse the removal of part of the invariant chain (CLIP) from the MHC class II αβ–CLIP complex and to facilitate antigenic peptide loading to class II molecules. In H2-M (the mouse equivalent of DM)-deficient mice, normal amounts of class II molecules are present on the cell surface, but most of them are associated with CLIP peptides; the CD4+ T cells from the deficient mice failed to respond to self-antigen-presenting cells (APC), but reacted strongly to wild-type APC [8]. Discordant regulation of HLA-DM, invariant chain (Ii) and class II molecules may enable HLA class II molecules to present novel self-peptides to which the T cells are not tolerant [9]. Although a number of studies have been done on MHC class II expression in autoimmune diseases [10], to our knowledge no work has been published on HLA-DM expression on the autoimmune target cells.
Though it is generally accepted that autoimmunity results from the failure of peripheral T cell tolerance, there is controversy over the role of class II expression on non-professional APC, such as thyrocytes, in inducing T cell (auto) reactivity [11]. In this study, we wished to determine whether thyrocytes express the molecules known to be necessary for antigen processing and hence possibly presentation.
HLA class II data with primary thyrocyte cultures are often questioned on the basis of contamination with other infiltrating cells, while results from transformed or tumour cell lines may not represent the situation in normal or autoimmune thyrocytes [12]. Attempts to establish proliferating human thyroid epithelial cell cultures from normal donors have, until recently, been unsuccessful [13–16]. Long-term cultures of differentiated proliferating thyrocytes, which are not subject to ‘spontaneous’ transformation in vitro from normal individuals, have now been developed [17]. We have a pure thyrocyte strain, HTV-59A, with a highly specific medium which makes possible the detailed investigation of HLA-DMB, Ii, DRA and CIITA gene expression on differentiated thyroid epithelial cells.
We have characterized and then assessed HTV-59A for the presence of HLA-DMB, Ii, DRA and CIITA in basal and in interferon-gamma (IFN-γ)-induced conditions. For comparison, an SV-40 transfected thyrocyte clone, 3A10, which was cloned to homogeneity and is free of other contaminating cells, was also used in the study [18].
Here we provide evidence that DMB, Ii, CIITA and DRA can be expressed by thyrocytes, and, therefore, indicate that thyrocytes are capable of antigen processing and possibly antigen presentation to T cells.
MATERIALS AND METHODS
Cells
The normal thyroid epithelial cell strain HTV-59A was originally isolated from a patient with calcitonin-producing tumour [17]. As reported previously, the culture medium consists of a modified F-12 supplemented with bovine hypothalamus and pituitary extracts [17]. The cells were cultured in a 5% CO2 incubator at 37°C for a limited period (less than six passages), and the medium was changed every 3–5 days. Parallel cultures were grown in which half the cells were supplemented with 0.2–200 U/ml of recombinant human IFN-γ (Genzyme Diagnostics, Cambridge, MA) for between 0.5 and 4 days while the other half were grown without IFN-γ under the same conditions. The cells were then detached by incubation with 1 × trypsin/EDTA (Gibco BRL) and washed.
For comparison, the SV-40-transfected thyrocyte clone 3A10 was cultured with or without IFN-γ. CEM, an HLA class II− T lymphoblastoid cell line, and Hom-2, an HLA class II+ B lymphoblastoid cell line, were cultured in RPMI 1640 medium, plus 10% fetal calf serum (FCS), and served as negative or positive controls [19, 20].
Antibodies
The following antibodies were used to assess possible contamination by other cells and to characterize the HTV-59A thyrocytes: mouse anti-human MoAbs CD3–FITC, CD4–FITC, CD8–PE, CD19–FITC, CD16a–PE, CD56–PE and HLA-DR–PE were purchased from Becton Dickinson (San Jose, CA); MoAb LE61 was a gift from Dr B. Lane (University of Dundee, UK); UEA-1–FITC was obtained from Vector (Peterborough, UK), anti-calcitonin sera from Sera-Lab (Crawley Down, UK); and human anti-thyroid peroxidase (TPO) serum from our Laboratory. Rabbit anti-DMB serum was kindly provided by Dr P. Cresswell (Howard Hughes Medical Institute, Yale University School of Medicine, CT).
Immunofluorescence
For analysis by flow cytometry, freshly isolated HTV-59A thyrocytes were stained with antibodies for 30 min on ice. After washing, the cells were resuspended in PBS and analysed on a FACScan flow cytometer (Becton Dickinson).
For analysis by fluorescence microscopy, thyroid cells were cultured for 1–2 days on glass coverslips in medium containing 5% FCS to allow the cells to adhere. HTV-59A thyrocytes were stained with specific antibodies, washed and then incubated with second antibody (e.g. FITC-conjugated rabbit anti-mouse immunoglobulin). For staining with antibodies to cytokeratin, calcitonin and TPO, the cells were prefixed with cold methanol/acetone (1:1) for 10 min, while the others were postfixed with 95% ethanol/5% acetic acid only. After the coverslip was mounted onto microscope slides and sealed, analyses were carried out by counting at least 100 thyrocytes by phase contrast (for total cells) and fluorescence microscopy (for staining cells) at a magnification of × 400 on a Zeiss III RS microscope.
Reverse transcriptase-polymerase chain reaction analysis
RNA was prepared by a method adapted from Chomczynski & Sacchi [21]. Briefly, total RNA was isolated by treatment of washed cells with guanidinium lysis buffer, followed by extraction with phenol:chloroform. The quantity of RNA was determined using a DNA Dipstick Kit (Invitrogen, San Diego, CA). cDNA was synthesized from 5 μg of total RNA using the cDNA Cycle Kit (Invitrogen) according to the manufacturer's recommendations.
For polymerase chain reaction (PCR), cDNA (1–5 μl out of 20 μl preparations) was made up with 25 pmole of each PCR primer, 200-μm concentration of each deoxynucleoside triphosphate (Pharmacia), 1 × reaction buffer (Appligene, Oncor, France) and 5–10 U of Taq polymerase (Appligene) in a 50-μl reaction volume. The forward and reverse primers and the expected PCR product sizes (in parentheses) are as follows: β-actin, 5′ CACCCCGTGCTGCTGACCGAGGCC 3′ and 5′ CCAC ACGGAGTACTTGCGCTCAGG 3′ (720 bp); DMB 5′ TCCTTCA ACAAGGATCTGCTG 3′ and 5′ CTTCCTCC ACGTGATAGTCAC 3′ (321 bp); DRA 5′ CGAGTTCTATCTGAATCCTG 3′ and 5′ GTTCTGCTGCATTGCTTTTGC 3′ (644 bp); Ii 5′ TCCCAAGC CTGTGAGCAAGATG 3′ and 5′ CCAGTTCCAGTGACTCTT TCG 3′ (341 bp); CIITA 5′ CAAGTCCCTGAAGGATGTGGA 3′ and 5′ ACGTCCATCACCCGGAGGGAC 3′ (471 bp). The amount of starting material (cDNA) and the numbers of PCR cycles were selected by pioneer experiments and samples of the PCR reactions were taken at multiple points throughout the amplification process for appropriate quantification. A negative control (without cDNA) was used to detect any false-positive product. PCR was performed in a DNA Thermal Cycler with 25–36 cycles of 94°C (1 min) → 55°C (2 min) → 72°C (3 min).
The PCR products were separated in 1% agarose gels containing ethidium bromide and photographed. A 100 base pair DNA ladder (Gibco BRL) was included for reference. The specificity of the PCR products was further checked by DNA sequencing using the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction Kit and ABI 373A DNA Sequencer (Applied Biosystems, Perkin-Elmer, Foster City, CA). The sequence data were analysed by Sequence Navigator and Auto Assembler software.
Western blot analysis
HTV-59A thyrocytes (0.5 × 106–2 × 106) were frozen at −80°C, and thawed and lysed with 2 × SDS sample buffer (0.125 m Tris–HCl pH 6.8, 6.8% SDS, 10% β-mercaptoethanol, 20% glycerol, 0.025% bromophenol blue) at 95°C for 5 min, and resolved on 10% SDS–PAGE gels with the rainbow coloured molecular weight marker (Amersham, Aylesbury, UK) before transfer to Immobilon P membranes (Millipore, Bedford, MA). Blots were blocked with 10% dry milk powder in Tris-buffered saline containing 0.1% Tween-20 before incubation with the polyclonal antiserum against DMB at a dilution of 1:2500. Immunoreactive protein was detected using the ECL Western blotting system (Amersham).
RESULTS
Characterization of HTV-59A thyrocytes
MoAbs to several surface antigens were used to assess possible contamination by lymphocytes within the HTV-59A thyrocyte preparation. As shown in Fig. 1, only 0.05% cells stained with CD3 and CD4 MoAbs, none with CD8 or CD16a; while 0.05% cells were positive for CD19. CD56, an antigen expressed on both natural killer (NK) cells and differentiated thyrocytes, was positive on 83% of the cells, as expected [22]. Therefore, the HTV-59A thyrocyte strain was pure with only negligible quantities of possible lymphocytes. To our knowledge, this is the most homogeneous and differentiated human thyrocyte strain available at present.
Fig 1.
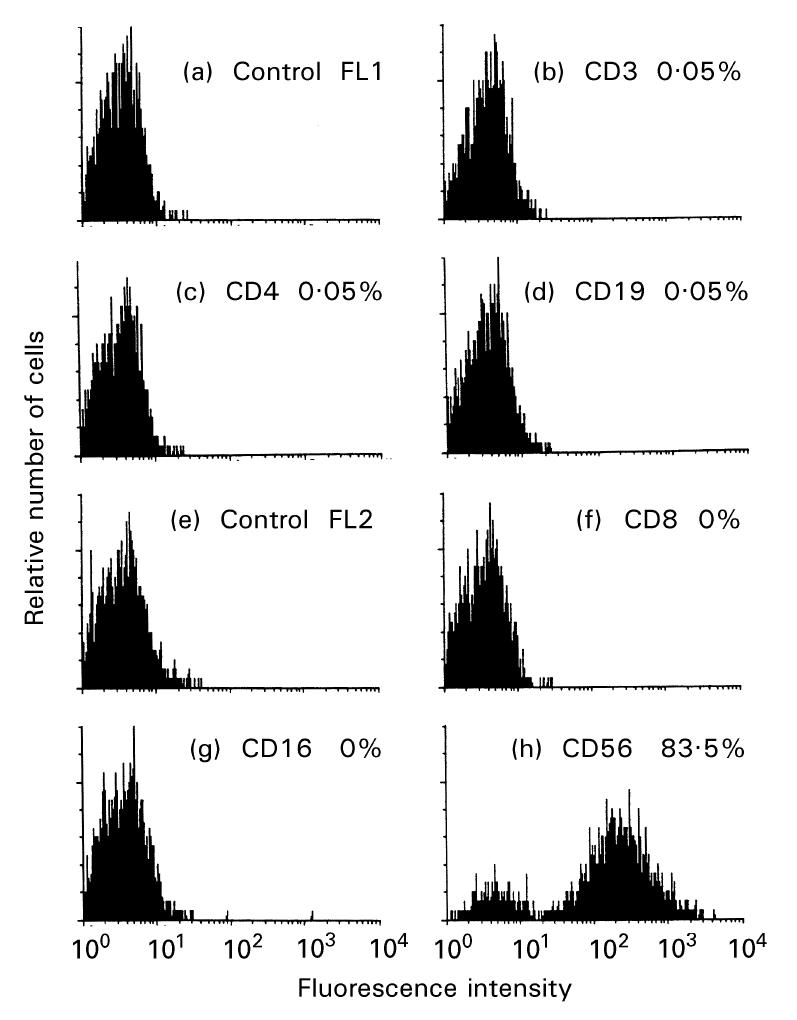
Flow cytometry analysis of CD3 (b), CD4 (c), CD8 (f), CD16 (g), CD19 (d) and CD56 (h) antigen expression on HTV-59A thyrocytes. Cells positive for a specific antigen are shown as percentages. Relative numbers of cells is indicated on the ordinate and the logarithm of fluorescence intensity is on the abscissa.
As seen in Fig. 2a,b, the cytokeratin skeleton of all cells stained strongly with LE-61, demonstrating the epithelial cell characteristics of HTV-59A thyrocytes. Indirect immunofluorescence (IFL) staining, with the anti-TPO serum on fixed monolayers of HTV-59A, showed cytoplasmic staining for TPO in virtually all of the cells. However, staining of the non-fixed cells showed only about 5% of the cells expressed surface antigen (Fig. 2c–f).
Fig 2.
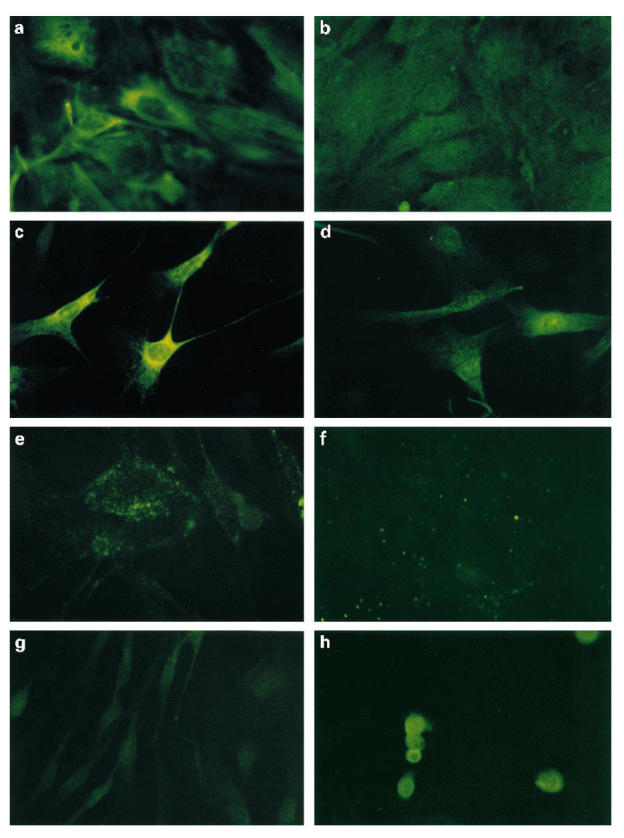
Indirect immunofluorescence staining of HTV-59A thyrocytes with: (a) LE-61 or (b) control P11; (c) anti-thyroid peroxidase (TPO) serum (cells prefixed with methanol/acetone) or (d) control sera (prefixed cells); (e) the anti-TPO serum (post fixed cells) or (f) with control sera; (g) anti-calcitonin antisera or (h) the antisera to control epithelial cell line RT-112 and immunofluorescence staining of HTV-59A thyrocytes with (see next page) (i) an endothelial cell marker UEA-1 or (j) with non-endothelial cell lectin–FITC (original mag. × 400).
As shown in Fig. 2g,h, no positive staining was observed with either anti-calcitonin antiserum or UEA-1 (an endothelial cell marker), indicating clearly that the HTV-59A thyrocytes were not contaminated with calcitonin-containing cells or endothelial cells. However, background staining appeared in the control RT-112 cell line with anti-calcitonin antibodies, as a result of the recognized effect of cell prefixation.
HTV-59A thyrocytes have a low level of spontaneous HLA-DR expression (Fig. 3). Treatment of the cells with IFN-γ (10 U/ml or more for 2–4 days) causes a uniform enhancement of cell-surface HLA-DR antigen expression in > 90% of cells. The dose and time courses of IFN-γ induction have been studied and further experiments also indicated that no significant changes to HLA-DR induction were observed when 10–500 U/ml IFN-γ were added to the culture medium for 2 days. Also, after IFN-γ stimulation, no change in the level of cell contamination was observed by staining with the above antibodies (both data not shown).
Fig 3.
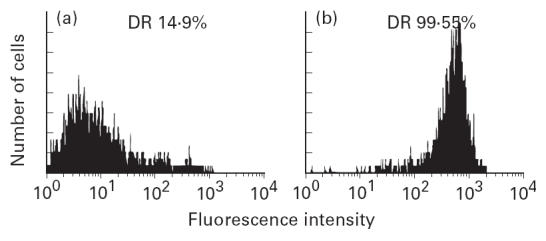
Flow cytometry analysis of HLA class II antigen expression on HTV-59A thyrocytes in the absence (a) or in the presence of IFN-γ 200 U/ml for 4 days (b). Thyrocytes were stained with anti-HLA-DR MoAb. DR+ cells are shown as a percentage.
HLA-DR expression in CEM, Hom-2 and 3A10 cells
CEM expressed no HLA-DR antigen, whereas Hom-2, the HLA class II+ cell control, expressed high levels of DR antigen (Fig. 4a–d). As shown in Fig. 4e,f, 3A10 has a basal expression of HLA-DR (about 17%), and is enhanced by IFN-γ.
Fig 4.
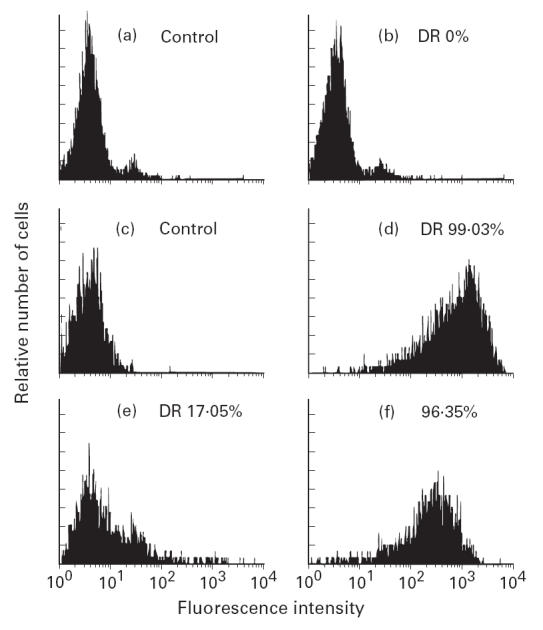
Flow cytometry analysis of HLA-DR antigen expression on CEM (b), Hom-2 (d) and SV-40-transfected thyrocyte clone 3A10 in the absence (e) and in the presence of IFN-γ 100 U/ml for 3 days (f).
Time course of DRA gene up-regulation in HTV-59A thyrocytes
A time-course analysis was performed with a dose of 200 U/ml of IFN-γ. In agreement with the results from our FACS analyses, DRA mRNA was present in HTV-59A thyrocytes in basal conditions (Fig. 5a, 0 h). The level of DRA mRNA started to increase after 6 h of IFN-γ treatment, reached its peak at 12 h and persisted for at least 48 h. The DRA amplification product was estimated at 650 base pairs, which was in good agreement with the distance (644 bp) between the DRA primers (Fig. 5a).
Fig 5.
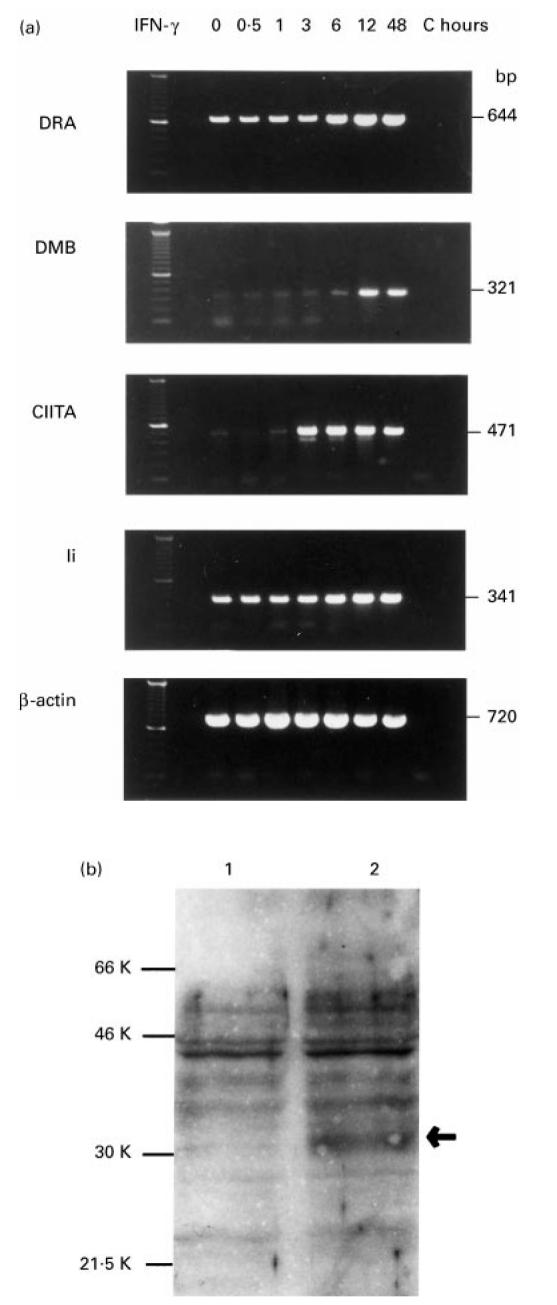
(a) Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of DRA, DMB, CIITA, and Ii mRNAs expression on HTV-59A thyrocytes. The thyrocytes were treated without or with IFN-γ 200 U/ml for 0.5, 1, 3, 6, 12 and 48 h. C indicates the reaction control (without cDNA). The product sizes are indicated on the right. (b) Western blot analysis of DMB expression. The DMB band (at an apparent molecular mass of 31 kD) was enhanced by IFN-γ treatment (lane 2).
Expression of DMB and Ii genes in HTV-59A thyrocytes
The HLA class II-like genes DMA and DMB regulate the antigen presentation function of MHC class II molecules. As shown in Fig. 5a, a low level of DMB transcripts was detectable under basal conditions (0 h), was enhanced at 6 h after IFN-γ treatment, peaked at 12 h and remained unchanged for at least 48 h after treatment. The PCR product for DMB appeared as a single band on agarose gels between 300 and 400 base pairs in size, which is in agreement with the expected size of 321 base pairs.
The invariant chain associates with class II molecules shortly after their synthesis and plays a central role in regulating class II expression and function. Figure 5a shows that mRNA for Ii was detected in basal conditions (0 h), enhanced expression of Ii mRNA was observed after 6 h and that Ii mRNA levels peaked 12 h after treatment. Expression of Ii, DMB and DRA all showed a similar pattern: increased expression was observed at 6 h and peaked 12 h after IFN-γ treatment. These results indicate that DMB expression was up-regulated by IFN-γ in a way similar to Ii and DRA in these cells.
DMB protein enhanced in HTV-59A thyrocytes
To examine protein expression of DMB in HTV-59A cells, Western blot analysis using the anti-DMB rabbit antiserum was performed. A faint band at an apparent molecular mass of 31 kD, corresponding to DMB protein [23], was detected in HTV-59A thyrocytes, and the level of the protein was enhanced after IFN-γ treatment for 2 days (Fig. 5b).
Effect of IFN-γ treatment on CIITA transcripts
CIITA is able to correct the MHC class II regulatory defect in some cases of bare lymphocyte syndrome and is important for MHC class II gene expression. However, the copy number of CIITA message is low relative to DRA [24]. As shown in Fig. 5a, a low level of CIITA mRNA expression in HTV-59A cells was observed without IFN-γ stimulation. Treatment with IFN-γ rapidly increased CIITA mRNA expression, which rose as early as 3 h after treatment to almost maximum levels and was unchanged at 48 h. These results demonstrated that CIITA mRNA expression in HTV-59A thyrocytes was up-regulated by IFN-γ and that the expression of the CIITA transcript preceded that of DMB, Ii and DRA mRNAs, indicating that CIITA itself might regulate MHC class II gene expression.
The PCR products for DRA, DMB, Ii, CIITA and β-actin were further checked by DNA sequence analysis. The sequence data confirm the specificity of the products for individual genes (data not shown).
Expression of DRA, DMB, Ii and CIITA genes in CEM, Hom-2 and 3A10 cells
As shown in Fig. 6, DRA mRNA was barely detectable by RT-PCR analysis in CEM cells, which was consistent with their class II− status (lane 1). In contrast, DRA mRNA was clearly evident in the Hom-2 cell line (lane 2). In the SV-40-transfected thyrocytes, DRA mRNA was observed in the cells in basal conditions, as expected (lane 3), and the mRNA level increased after IFN-γ treatment (lane 4).
Fig 6.
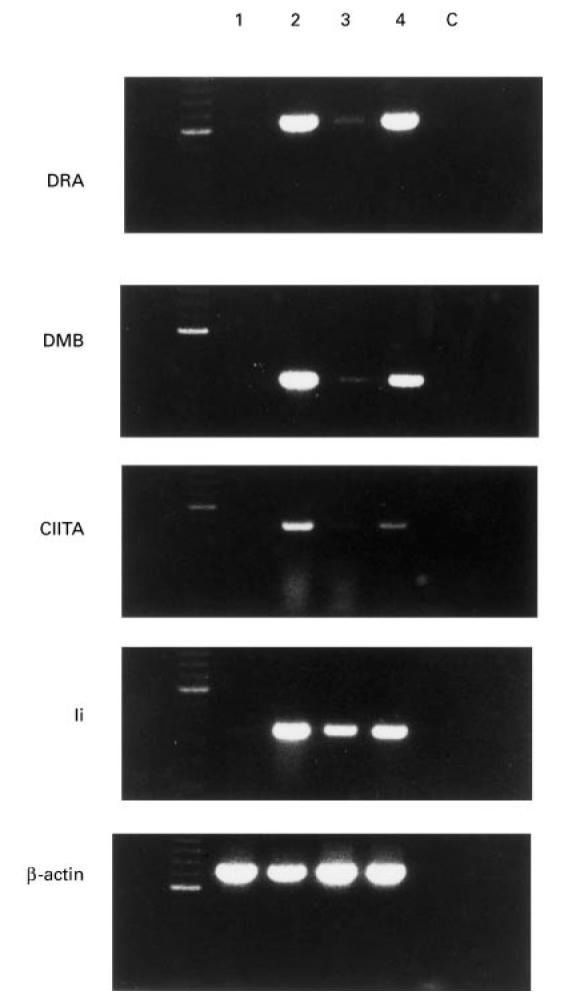
Expression of DRA, DMB, CIITA and Ii mRNAs in CEM, Hom-2 and SV-40-transfected thyrocytes. Reverse transcriptase-polymerase chain reactions (RT-PCR) were performed on RNAs prepared from CEM (lane 1), Hom-2 (lane 2), SV-40-transfected thyrocytes in the absence of (lane 3) or in the presence of IFN-γ (lane 4). C is the PCR negative control.
DMB and Ii transcripts were detected at very low levels in the class II− CEM cells, though the bands do not appear on the present illustrations. Much higher levels were observed in class II+ Hom-2 cells (lane 2). In the SV-40-transfected thyrocytes, 3A10, constitutive expression of DMB and Ii mRNAs were seen under basal conditions (lane 3), and enhanced expression of the transcripts was observed upon treatment with IFN-γ.
CIITA mRNA was not detectable in CEM cells even though 5 μl of cDNA were used and the PCR reaction was performed for 30 cycles. By contrast, CIITA was identified in Hom-2 cells. In SV-40-transfected thyrocytes, a low level of CIITA mRNA was observed in the cells in basal conditions, while enhanced expression was evident after IFN-γ treatment.
DISCUSSION
Normal human thyroid cells, kept in long-term cultures under the conditions used here, are not subject to ‘spontaneous’ transformation in vitro [17]. The characterization of HTV-59A showed that the cells are pure thyrocytes with negligible numbers of lymphocytes (0.05–0.1%). In agreement with a previous report on normal thyrocytes [25], HTV-59A thyrocytes also show some basal HLA class II expression which may be enhanced by thyroid-stimulating hormone (TSH) in the culture medium [17, 26]. Therefore, HTV-59A cells are suitable material for the study of DMB expression and transcriptional regulation of class II genes in differentiated thyrocytes.
The present study demonstrates that HLA-DMB and Ii transcripts are consistently present at low levels in HTV-59A and are enhanced by IFN-γ treatment. The up-regulation of class II genes requires de novo protein synthesis as cyclohexamide, an inhibitor of protein synthesis, prevents IFN-γ-induced expression of class II [27, 28]. The fact that expression of DMB, Ii and DRA after IFN-γ treatment all show a similar time course may reflect the same regulatory mechanism: i.e. de novo protein synthesis is needed for IFN-γ stimulation.
As discussed previously, primary thyroid cell cultures are usually contaminated by a small proportion of other cells. Hence the expression of DM, Ii and DRA in primary thyrocyte cultures could be a result of local cytokine production. In this purified thyrocyte strain, no obvious evidence of contamination by other cells could be observed. The expression of DMB, Ii, DRA and CIITA observed in the SV-40-transfected clone, 3A10, is totally consistent with the results from HTV-59A, and demonstrates that thyrocytes do express the genes important for antigen processing and presentation.
The observation that the promoter regions of DMA and DMB are similar to those of the class II genes indicates that DM may also be regulated by CIITA [5]. The DM genes are regulated by a mechanism common to the HLA class II genes, through the conserved X-Y-Z promoter region and the transcription factors CIITA and RFX [29]. In HTV-59A, the increase of CIITA mRNA after IFN-γ treatment occurred considerably earlier than DMB, Ii, and DRA induction. This is consistent with its requirement for DMB, Ii and DRA expression, since CIITA is first translated into protein that itself induces class II mRNA.
Similar to BLS-2 cells [30], very low levels of DMB and Ii transcripts were detected in the class II− cell line CEM, whereas no mRNAs for CIITA and DRA were identified, though the reason for the presence of DMB and Ii mRNAs in CEM is not clear.
The data presented here indicate the possible involvement of DMB in antigen processing and presentation in thyroid epithelial cells. Previous studies have shown that class II-expressing thyrocytes could stimulate the proliferation of autologous T cells grown from autoimmune thyrocyte glands [31–34]. These studies concluded that local up-regulation of antigen-presenting function on thyrocytes may have been an early event in the development of AITD [34]. Autoantigen presentation by thyrocytes is more efficient than by professional APC, because the thyroid autoantigens (TPO and TSH-R) are membrane proteins and would be expected to be recycled and presented by class II molecules, and the effective concentration of these antigens on thyroid epithelial cells may be higher than when picked up by other APC [10, 35].
Lack of expression of costimulators such as B7-1 or B7-2 by thyrocytes undermines their ability to present antigen. Instead, class II-expressing thyrocytes may induce tolerance in autoreactive CD4+ T cells [36, 37]. However, recent studies using fibroblasts showed that immunization of mice with the cells transfected with both a class II molecule and the human thyrotropin receptor, but not by either alone, induced a Graves'-like disease and that immunizing mice with fibroblasts transfected with a viral protein could induce a cytotoxic T cell response without costimulators, suggesting that costimulatory signals could either be provided by bystander professional APC or be host-derived [37–39].
Our results with CIITA, DMB and Ii expression only indicate that thyrocytes have the potential for antigen presentation but do not provide evidence for functional antigen-presenting ability in an autoimmune process. Diverse roles for class II expression on thyrocytes may exist: class II molecules expressed on normal cells may contribute to peripheral tolerance whereas class II on thyrocytes in AITD could facilitate recognition by autoreactive T cells, break peripheral tolerance and exacerbate the disease [10, 40]. Professional APC such as dendritic cells also play a vital role in presenting thyroid autoantigens and providing costimulatory molecules in AITD. Similar to HLA class I and transporter associated with antigen processing (TAP)-1 expression, the hyperinducibility of class II genes in thyrocytes from GD has been thought to be due to a breach of peripheral tolerance [41, 42]. However, with the theoretical models of Matzinger, this concept has to be reviewed and it would be critical to understand what the costimulatory signals are and how dendritic cells, lymphocytes and thyrocytes interact in AITD as a whole [43–45].
Acknowledgments
We wish to thank Dr Peter Cresswell for providing HLA-DMB antibody and Dr Yunliang Chen for his technical help. Z.W., L.H., and this work were supported by the Autoimmune Diseases Charitable Trust. The work was also supported by grants from the ASI (Agenzia Spaziale Italiana), CNR (Consiglio Nazionale delle Ricerche, P.F. Oncologia) and MURST (Ministero dell'Universita e della Ricerca Scientifica e Technologica).
References
- 1.McGregor AM. Autoimmunity in the thyroid—can the molecular revolution contribute to our understanding? Q J Med. 1992;82:1–13. [PubMed] [Google Scholar]
- 2.Bernet V, Burman K. Autoimmune thyroid disease principles and practice. In: Rich RR, Fleisher TA, Schwartz BD, Shearer WT, Strober W, editors. Clinical immunology. St Louis, MO: Mosby; 1996. pp. 1482–6. [Google Scholar]
- 3.Mach B, Steimle V, Martinez-Soria E, Reith W. Regulation of MHC class II genes: lessons from a disease. Annu Rev Immunol. 1996;14:301–31. doi: 10.1146/annurev.immunol.14.1.301. [DOI] [PubMed] [Google Scholar]
- 4.Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–99. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 5.Kelly AP, Monaco JJ, Cho SG, Trowsdale J. A new human HLA class II-related locus, DM. Nature. 1991;353:571–3. doi: 10.1038/353571a0. [DOI] [PubMed] [Google Scholar]
- 6.Morris P, Shaman J, Attaya M, et al. An essential role for HLA-DM in antigen presentation by class II major histocompatibility molecules. Nature. 1994;368:551–4. doi: 10.1038/368551a0. [DOI] [PubMed] [Google Scholar]
- 7.Fling SP, Arp B, Pious D. HLA-DMA and -DMB genes are both required for MHC class II/peptide complex formation in antigen-presenting cells. Nature. 1994;368:554–8. doi: 10.1038/368554a0. [DOI] [PubMed] [Google Scholar]
- 8.Fung-Leung WP, Surh CD, Liljedahl M, et al. Antigen presentation and T cell development in H2-M-deficient mice. Science. 1996;271:1278–81. doi: 10.1126/science.271.5253.1278. [DOI] [PubMed] [Google Scholar]
- 9.Lightstone L, Hargreaves R, Bobek G, et al. In the absence of the invariant chain, HLA-DR molecules display a distinct array of peptides which is influenced by the presence or absence of HLA-DM. Proc Natl Acad Sci USA. 1997;94:5772–7. doi: 10.1073/pnas.94.11.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todd I, Bottazzo GF. On the issue of inappropriate HLA class II expression on endocrine cells: an answer to a sceptic. J Autoimmun. 1995;8:313–22. doi: 10.1006/jaut.1995.0024. [DOI] [PubMed] [Google Scholar]
- 11.Miller JF, Flavell RA. T-cell tolerance and autoimmunity in transgenic models of central and peripheral tolerance. Curr Opin Immunol. 1994;6:892–9. doi: 10.1016/0952-7915(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 12.Weetman AP. Autoimmune thyroiditis: predisposition and pathogenesis. Clin Endocrinol (Oxf) 1992;36:307–23. doi: 10.1111/j.1365-2265.1992.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 13.Roger PP, Dumont JE. Thyrotropin is a potent growth factor for normal human thyroid cells in primary culture. Biochem Biophys Res Commun. 1987;149:707–11. doi: 10.1016/0006-291x(87)90425-6. [DOI] [PubMed] [Google Scholar]
- 14.Raspe E, Andry G, Dumont JE. Adenosine triphosphate, bradykinin, and thyrotropin-releasing hormone regulate the intracellular Ca2+ concentration and the 45Ca2+ efflux of human thyrocytes in primary culture. J Cell Physiol. 1989;140:608–14. doi: 10.1002/jcp.1041400328. [DOI] [PubMed] [Google Scholar]
- 15.Raspe E, Laurent E, Andry G, Dumont JE. ATP, bradykinin, TRH and TSH activate the Ca(2+)-phosphatidylinositol cascade of human thyrocytes in primary culture. Mol Cell Endocrinol. 1991;81:175–83. doi: 10.1016/0303-7207(91)90216-f. [DOI] [PubMed] [Google Scholar]
- 16.Van Sande J, Raspe E, Perret J, et al. Thyrotropin activates both the cyclic AMP and the PIP2 cascades in CHO cells expressing the human cDNA of TSH receptor. Mol Cell Endocrinol. 1990;74:R1–R6. doi: 10.1016/0303-7207(90)90209-q. [DOI] [PubMed] [Google Scholar]
- 17.Curcio F, Ambesi-Impiombato FS, Perrella G, Coon HG. Long-term culture and functional characterization of follicular cells from adult normal human thyroids. Proc Natl Acad Sci USA. 1994;91:9004–8. doi: 10.1073/pnas.91.19.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belfiore A, Mauerhoff T, Pujol-Borrell R, et al. De novo HLA class II and enhanced HLA class I molecule expression in SV40 transfected human thyroid epithelial cells. J Autoimmun. 1991;4:397–414. doi: 10.1016/0896-8411(91)90155-6. [DOI] [PubMed] [Google Scholar]
- 19.Foley GE, Lazarus H, Farber S, Uzman BG, Boone BA, McCarthy RE. Continuous culture of human lymphoblasts from peripheral blood of a child with acute leukemia. Cancer. 1965;18:522–9. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 20.Williams KM, Raybourne RB. Demonstration of cross-reactivity between bacterial antigens and class I human leukocyte antigens by using monoclonal antibodies to Shigella flexneri. Infect Immun. 1990;58:1774–81. doi: 10.1128/iai.58.6.1774-1781.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 22.Migita K, Eguchi K, Kawakami A, et al. Detection Leu-19 (CD56) antigen on human thyroid epithelial cells by an immunohistochemical method. Immunology. 1991;72:246–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Denzin LK, Robbins NF, Carboy-Newcomb C, et al. Assembly and intracellular transport of HLA-DM and correction of the class II antigen-processing defect in T2 cells. Immunity. 1994;1:595–606. doi: 10.1016/1074-7613(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 24.Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–46. [PubMed] [Google Scholar]
- 25.Lucas-Martin A, Foz-Sala M, Todd I, Bottazzo GF, Pujol-Borrell R. Occurrence of thyrocyte HLA class II expression in a wide variety of thyroid diseases: relationship with lymphocytic infiltration and thyroid autoantibodies. J Clin Endocrinol Metab. 1988;66:367–75. doi: 10.1210/jcem-66-2-367. [DOI] [PubMed] [Google Scholar]
- 26.Todd I, Pujol-Borrell R, Hammond LJ, McNally JM, Feldmann M, Bottazzo GF. Enhancement of thyrocyte HLA class II expression by thyroid stimulating hormone. Clin Exp Immunol. 1987;69:524–31. [PMC free article] [PubMed] [Google Scholar]
- 27.Blanar MA, Boettger EC, Flavell RA. Transcriptional activation of HLA-DR alpha by interferon gamma requires a trans-acting protein. Proc Natl Acad Sci USA. 1988;85:4672–6. doi: 10.1073/pnas.85.13.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bottger EC, Blanar MA, Flavell RA. Cycloheximide, an inhibitor of protein synthesis, prevents gamma-interferon-induced expression of class II mRNA in a macrophage cell line. Immunogenetics. 1988;28:215–20. doi: 10.1007/BF00345497. [DOI] [PubMed] [Google Scholar]
- 29.Westerheide SD, Louis-Plence P, Ping D, He XF, Boss JM. HLA-DMA and HLA-DMB gene expression functions through the conserved S-X-Y region. J Immunol. 1997;158:4812–21. [PubMed] [Google Scholar]
- 30.Chang CH, Flavell RA. Class II transactivator regulates the expression of multiple genes involved in antigen presentation. J Exp Med. 1995;181:765–7. doi: 10.1084/jem.181.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Londei M, Lamb JR, Bottazzo GF, Feldmann M. Epithelial cells expressing aberrant MHC class II determinants can present antigen to cloned human T cells. Nature. 1984;312:639–41. doi: 10.1038/312639a0. [DOI] [PubMed] [Google Scholar]
- 32.Londei M, Bottazzo GF, Feldmann M. Human T-cell clones from autoimmune thyroid glands: specific recognition of autologous thyroid cells. Science. 1985;228:85–89. doi: 10.1126/science.3871967. [DOI] [PubMed] [Google Scholar]
- 33.Weetman AP, Volkman DJ, Burman KD, et al. The production and characterization of thyroid-derived T-cell lines in Graves' disease and Hashimoto's thyroiditis. Clin Immunol Immunopathol. 1986;39:139–50. doi: 10.1016/0090-1229(86)90213-8. [DOI] [PubMed] [Google Scholar]
- 34.Dayan CM, Londei M, Corcoran AE, et al. Autoantigen recognition by thyroid-infiltrating T cells in Graves disease. Proc Natl Acad Sci USA. 1991;88:7415–9. doi: 10.1073/pnas.88.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldmann M, Dayan C, Rapoport B, Londei M. T cell activation and antigen presentation in human thyroid autoimmunity. J Autoimmun. 1992;5(Suppl. A):115–21. doi: 10.1016/0896-8411(92)90026-m. [DOI] [PubMed] [Google Scholar]
- 36.Tandon N, Metcalfe RA, Barnett D, Weetman AP. Expression of the costimulatory molecule B7/BB1 in autoimmune thyroid disease. Q J Med. 1994;87:231–6. [PubMed] [Google Scholar]
- 37.Matsuoka N, Eguchi K, Kawakami A, et al. Lack of B7-1/BB1 and B7-2/B70 expression on thyrocytes of patients with Graves' disease. Delivery of costimulatory signals from bystander professional antigen-presenting cells. J Clin Endocrinol Metab. 1996;81:4137–43. doi: 10.1210/jcem.81.11.8923872. [DOI] [PubMed] [Google Scholar]
- 38.Shimojo N, Kohno Y, Yamaguchi K, et al. Induction of Graves-like disease in mice by immunization with fibroblasts transfected with the thyrotropin receptor and a class II molecule. Proc Natl Acad Sci USA. 1996;93:11074–9. doi: 10.1073/pnas.93.20.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kundig TM, Bachmann MF, DiPaolo C, et al. Fibroblasts as efficient antigen-presenting cells in lymphoid organs. Science. 1995;268:1343–7. doi: 10.1126/science.7761853. [DOI] [PubMed] [Google Scholar]
- 40.Weetman AP. New aspects of thyroid immunity. Horm Res. 1997;48(Suppl. 4):51–54. doi: 10.1159/000191314. [DOI] [PubMed] [Google Scholar]
- 41.Sospedra M, Tolosa E, Armengol P, et al. Hyperexpression of transporter in antigen processing-1 (TAP-1) in thyroid glands affected by autoimmunity: a contributing factor to the breach of tolerance to thyroid antigens? Clin Exp Immunol. 1997;109:98–106. doi: 10.1046/j.1365-2249.1997.3811277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sospedra M, Obiols G, Babi LF, et al. Hyperinducibility of HLA class II expression of thyroid follicular cells from Graves' disease. A primary defect? J Immunol. 1995;154:4213–22. [PubMed] [Google Scholar]
- 43.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 44.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–6. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 45.Ridge JP, Rosa FD, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]


