Abstract
Oxidized low-density lipoprotein (oxLDL) consists of both lipid components and apoprotein B100. OxLDL has both proinflammatory and cytotoxic properties. The present study was undertaken to investigate the effects of components in the lipid moiety of oxLDL on immune activation as determined by cytokine and immunoglobulin secretion. LPC induced interferon-gamma (IFN-γ) secretion in peripheral blood mononuclear leucocytes from healthy blood donors. The effect varied between individuals, and there were both responders and non-responders. Furthermore, LPC induced enhanced antibody production, indicating B cell activation. None of eight oxysterols, arachidonic acid (AA), or 15-lipoxygenase products of AA tested had immune stimulatory properties. We recently demonstrated that PAF and oxLDL induce IFN-γ secretion by a common mechanism. LPC-induced IFN-γ secretion was inhibited by a specific PAF receptor antagonist, WEB 2170, indicating that the PAF receptor is involved in LPC-induced immune activation. Both oxLDL- and LPC-induced antibody formation was inhibited by WEB 2170. Furthermore LPC also induced tumour necrosis factor-alpha secretion, and this effect was inhibited by WEB 2170. LPC is produced during lipid oxidation (as in oxLDL), but also by enzymes such as phospholipase A2. The findings indicate that LPC may play an important role in inflammatory reactions, including atherosclerosis.
Keywords: atherosclerosis, cytokines, oxidized LDL, lysophosphatidylcholine, platelet-activating factor
INTRODUCTION
The oxidized form of LDL (oxLDL) may play an important role in the chronic inflammation characterizing atherosclerosis, since it is chemotactic and induces foam cell formation in monocytes/macrophages [1,2]. It enhances the adhesion of leucocytes to the endothelium [3], activates and differentiates monocytes/macrophages [4,5] and is immunogenic [6–10]. Recently, PAF has been shown to play a role both in oxLDL-induced endothelial–monocyte interactions [11] and oxLDL-induced immune activation [12]. The importance of oxLDL in atherosclerosis is underlined by the presence oxLDL in the atherosclerotic lesion [13], and anti-oxidants such as BHT inhibit development of atherosclerosis in experimental animals [14]. Comparatively little is known about which components of oxLDL are related to immune reactions seen in atherosclerosis. OxLDL consists of lipids, including cholesterol and oxysterols, phospholipids, oxidized fatty acids, aldehydes and lipid peroxides [15]. Apo B100 is the protein component of LDL and it binds to the LDL receptor [16]. During oxidation of LDL, apo B100 is fragmented and consequently LDL no longer binds to the LDL receptor. Instead it is taken up by a specific group of scavenger receptors on monocytes/macrophages [17]. LPC, which is a quantitatively important component of oxLDL, has been demonstrated to be a chemoattractant to monocytes [18] and T lymphocytes [19]. Furthermore, LPC induces adhesion molecules and mimics the proadhesive properties of oxLDL [20], and we recently demonstrated antibodies to LPC and showed that they cross-react with oxLDL [21]. In the present study we report that exposure to LPC, in contrast to other lipid components of oxLDL, induces immune activation by a PAF receptor-dependent mechanism. The relevance of these findings in atherosclerosis is discussed.
MATERIALS AND METHODS
Cell culture
Peripheral blood mononuclear cells (PBMC) were isolated from human buffy coats obtained from normal blood donors by sterile technique. The buffy coat was diluted in PBS at a ratio of 1:4, layered onto Ficoll–Hypaque and centrifuged for 20 min at 500 g. Cells at the interface were washed twice in PBS, counted and then resuspended in RPMI 1640 medium supplemented with HEPES buffer, penicillin and streptomycin and 10% fetal calf serum (FCS) (complete medium) at a cell concentration of 1 × 106 cells/ml.
Lipids and reagents
l-α-LPC (from egg yolk type 1), l-α-LPC 16:0 (synthetic), l-α-lysophosphatidylserine (from bovine brain), l-α-phosphatidylcholine,β-acetyl-γ-alkyl (PAF) were obtained from Sigma (Stockholm, Sweden). Oxysterols were obtained from Steraloids Inc. (Wilton, NH). The following oxysterols were used: 7α-hydroxycholesterol (5-cholesten-3β,7α -diol), 7β-hydroxycholesterol (5-cholesten-3β,7β-diol), α-epoxide (cholestan-5α,6α-epoxy-3β-ol), 3,5 diene (3,5 cholestadiene-7-one), cholestane triol (cholestane3β,5α,6β-triol), 25-hydroxycholesterol (5-cholesten-3β,25-diol), 7-ketocholesterol (5-cholesten-3β-ol-7-one), β-epoxide (cholestan-5β,6β-epoxy-3β-ol). Lipoxygenase products were obtained from Biomol Research Labs Inc. (Plymouth Meeting, PA). They included 15(S)-HETE, 15(S)-HPETE, 12(S)-HETE, 12(S)-HPETE, 13(S)-HODE, arachidonic acid and leukotriene B4.
All lipid substances were diluted in 70% ethanol. The final ethanol concentration in the experiments did not exceed 1%. WEB 2170, a specific PAF receptor antagonist, was from Boehringer-Ingelheim (Munich, Germany) [22].
Detection of interferon-gamma production
The ELISPOT technique was used to determine the frequency of cells producing interferon-gamma (IFN-γ) essentially as described [12,23]. Briefly, PBMC were suspended in complete medium at a concentration of 1 × 106 cells/ml at 37°C, 5% CO2 and exposed to different stimuli as indicated. Each measurement was performed in triplicate. After 48 h of culture 100 μl of the cell suspension were transferred to anti-IFN-γ-coated, 96-well nitrocellulose plates (Millititre HA; Millipore Co., Bedford, MA) for another 24 h incubation. The nitrocellulose plates were then washed twice and 100 μl of a detector MoAb (7-B6-1-Biotin; Mabtech AB, Stockholm, Sweden) at a concentration of 1 μl/ml were then added to each well in PBS. Thereafter the plates were kept at 4°C overnight. After washing, 100 μl of streptavidin-alkaline phosphatase (Mabtech AB) diluted at 1:1000 in PBS were added for 2 h at room temperature. After three more cycles of washing, 100 μl of BCIP-NBT substrate solution (BioRad, Richmond, CA) were added for 1 h at room temperature. Thereafter, the plates were washed three times with distilled water and left to dry at room temperature. The frequency of cells producing IFN-γ was determined. Spots were counted under low magnification (× 25) using an inverted microscope, each spot representing one IFN-γ-secreting cell.
Detection of immunoglobulin production
PBMC from healthy donors were cultured in complete medium at a concentration of 106 cells/ml. Cell suspensions were incubated with reagents as indicated. After 16 h the cells were washed three times in PBS and 100-μl cell suspensions at a concentration of 105/well were transferred to the precoated ELISPOT wells. The frequency of cells producing immunoglobulins was determined using a modified version of the ELISPOT technique as above and as recently described from our laboratory [24,25]. Spots were counted under low magnification (×2 5) using an inverted microscope.
Determination of tumour necrosis factor-alpha production
For determination of tumour necrosis factor-alpha (TNF-α) with ELISA, whole blood from healthy blood donors was diluted to 1:4 with serum-free RPMI 1640, while kept at 37°C, and then incubated with the indicated compounds for 24 h. An ELISA kit from Medgenix (Fleurus, Belgium) was used according to the manufacturer's instructions. Cell viability was determined by trypan blue exclusion and exceeded 95% in all experiments.
Statistical analysis
Conventional methods were used for calculation of means and s.d. Comparisons between control samples and samples stimulated with lipid derivates were analysed by computation of Student's t-test.
RESULTS
IFN-γ production at single-cell level
To allow a quantitative determination of the induction of cytokines at the single-cell level, the ELISPOT assay was used. There was an interindividual variation in IFN-γ secretion both without and with stimulation with LPC, which was present also when known antigens such as tetanus toxoid were used (data not shown). IFN-γ stimulatory properties of LPC were detected. At concentrations between 0.1 and 50 μm, LPC had the capacity to induce IFN-γ, and in general, maximal response occurred between 1 and 25 μm. At concentrations of 50 μm or higher, LPC tended to inhibit IFN-γ secretion (data not shown). Both LPC from egg yolk, containing also unsaturated fatty acids, and LPC:16, with only saturated fatty acids, enhanced IFN-γ secretion, indicating that oxidation is not a prerequisite for the effects described here. The capacity of LPC and LPC:16 to induce enhanced IFN-γ secretion is demonstrated in Fig. 1. Both oxLDL and LPC could induce IFN-γ in the same individuals, as shown in Fig. 2.
Fig. 1.
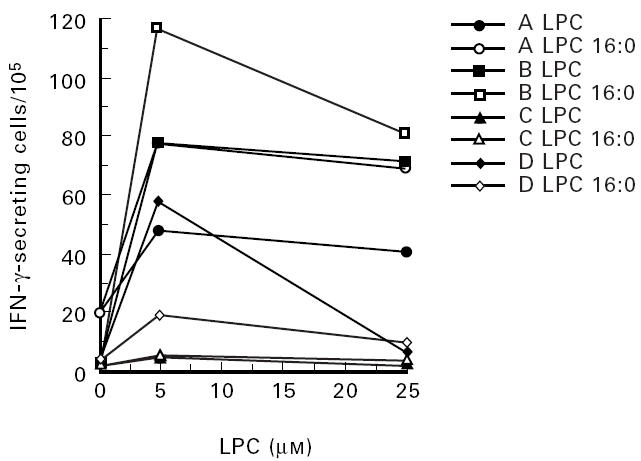
Effect of LPC on the secretion of IFN-γ. Peripheral blood mononuclear cells (PBMC) from four different donors (A, B, C, D) was grown in culture medium with the addition of LPC or LPC 16:0 at the indicated concentrations for 72 h. The frequency of IFN-γ-producing cells was determined by ELISPOT as indicated in Materials and Methods. Each value represents the mean of three determinations.
Fig. 2.
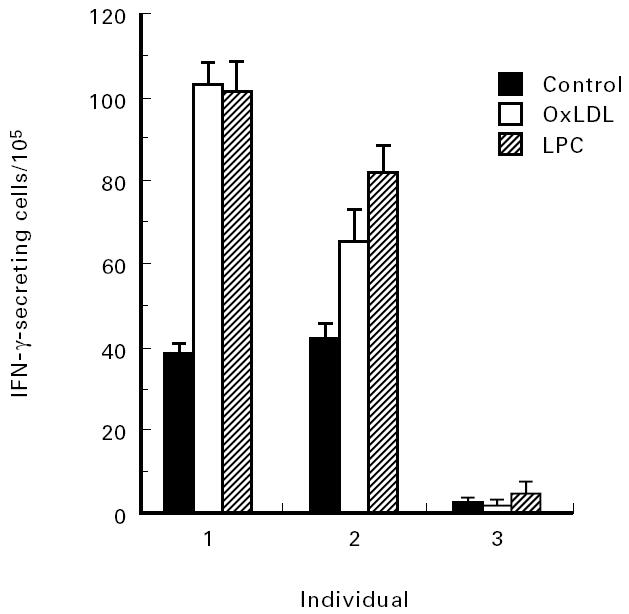
Effect of LPC and oxidized LDL on the secretion of IFN-γ. Peripheral blood mononuclear cells (PBMC) from three different donors was grown in culture medium with the addition LPC (5 μm) or oxidized low-density lipoprotein (oxLDL; 5 μg/ml) for 72 h. The frequency of IFN-γ-producing cells was determined by ELISPOT as indicated in Materials and Methods. Each value represents the mean of three determinations.
None of the eight oxysterols tested had any stimulatory effect on IFN-γ secretion at a concentration between 0.05 and 1 μm, and some of these tended to be inhibitory on IFN-γ secretion. At higher concentrations, the oxysterols were inhibitory or toxic, and at ≥ 5 μm the number of IFN-γ-secreting cells was below 50% of controls. None of the hydroxy or hydroperoxy fatty acids, including 15(S)-HETE, 15(S)-HPETE, 12(S)-HETE, 12(S)-HPETE, 13(S)-HODE, arachidonic acid and leukotriene B4, had any significant stimulatory effect on IFN-γ secretion at these concentrations (data not shown).
Characterization of LPC-induced IFN-γ production
In order to determine if LPC-induced IFN-γ secretion was mediated by the PAF receptor, we tested if a specific PAF receptor antagonist, WEB 2170, could inhibit the LPC-induced effects demonstrated here. We found that WEB 2170 inhibited both LPC- and PAF-induced IFN-γ secretion in all individuals tested (Fig. 3).
Fig. 3.
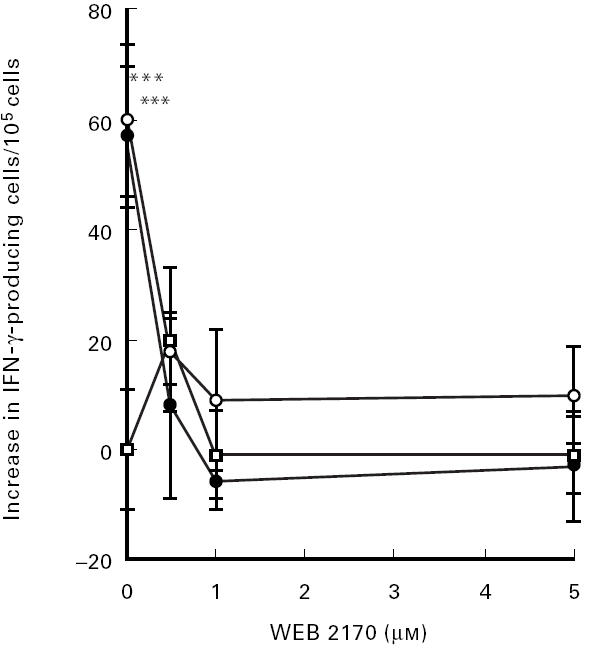
Effect of a specific PAF inhibitor (WEB 2170) on PAF- and LPC-induced IFN-γ secretion. Peripheral blood mononuclear cells (PBMC) were grown in culture medium with 1 μg/ml of PAF (•), 5 μm of LPC (○) or culture medium alone (□), with addition of WEB 2170 at the indicated concentrations for 72 h. Data are presented as increase in IFN-γ-producing cells/105 cells. Each value represents the mean ± s.d. of three determinations. ***P < 0.005 compared with basal level.
To investigate further the importance of the alcohol group of lysophospholipids on LPC-induced IFN-γ secretion, we tested the effects of lysophosphatidylserine compared with LPC. Our results indicate that LPC had a much stronger capacity than lysophosphatidylserine to induce IFN-γ (data not shown).
Effect of LPC on antibody secretion
The stimulatory effect of LPC on B cell activation was studied by determination of immunoglobulin production by PBMC. IgA showed the largest response of the immunoglobulins investigated. A representative experiment out of four in which LPC stimulated immunoglobulin formation as determined by the ELISPOT technique is depicted in Fig. 4. LPC-induced antibody formation was inhibited by WEB 2170 (Fig. 5).
Fig. 4.
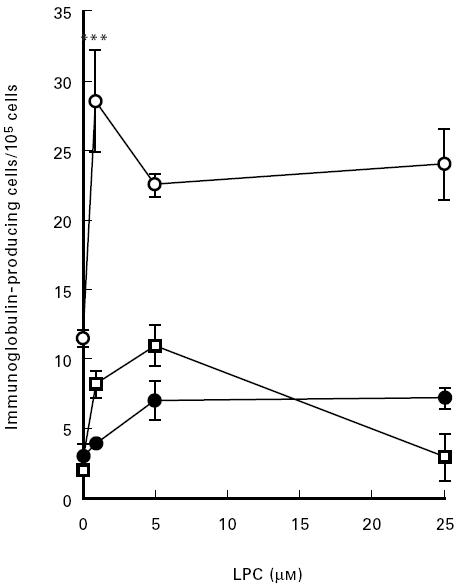
Effect of LPC on the secretion of IgG (□), IgA (○), or IgM (•). Peripheral blood mononuclear cells (PBMC) were grown in complete culture medium with the addition of LPC for 16 h. The frequency of immunoglobulin-producing cells was then determined by ELISPOT as indicated in Materials and Methods. Each value represents the mean ± s.d. of three determinations. ***P < 0.005.
Fig. 5.
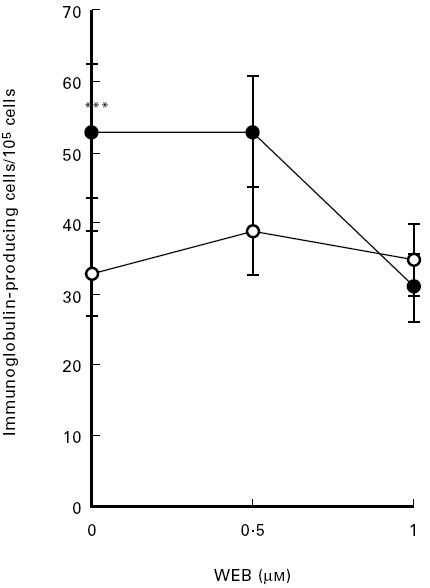
Effect of a specific PAF inhibitor (WEB 2170) on LPC-induced antibody formation. Peripheral blood mononuclear cells (PBMC) were grown in culture medium with 5 μm of LPC (•) or culture medium alone (○), with addition of WEB 2170 at the indicated concentrations for 72 h. Each value represents the mean ± s.d. of three determinations. ***P < 0.005 compared with basal level.
Effect of LPC and PAF on TNF-α secretion in peripheral blood
Whole blood was diluted with culture medium as described in Materials and Methods, and LPC and PAF were added at the indicated concentrations. After 24 h of culture, supernatants were obtained, centrifuged to eliminate cells and then TNF-α was determined by ELISA. The results show that LPC induced TNF-α secretion from cells in peripheral blood (Fig. 6a). LPC from egg yolk had a comparable capacity to synthetic LPC:16 to induce TNF-α secretion (data not shown). LPC-induced TNF-α secretion was abolished by WEB 2170 (Fig. 6b).
Fig. 6.
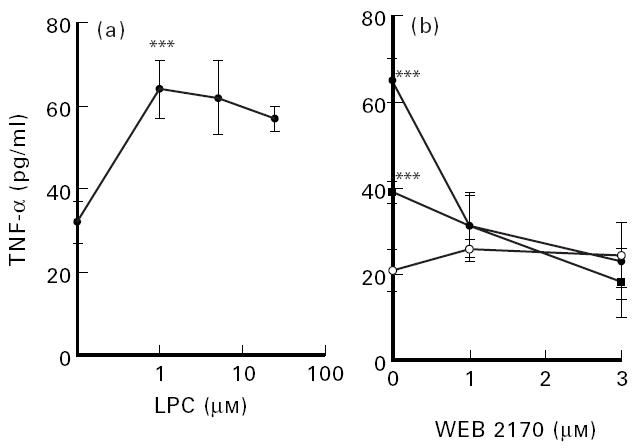
LPC-induced tumour necrosis-factor (TNF-α) secretion (a) and WEB 2170-induced inhibition of LPC-induced TNF-α secretion (b). Whole blood was diluted 1:4 in culture medium at 37°C as indicated in Materials and Methods. (b) LPC at 0 μm (○), 5 μm (•) or 25 μm (▪), and/or WEB 2170 were added at the indicated concentrations. The cells were then grown for 24 h in culture medium. TNF-α in the supernatant was then determined by ELISA as indicated in Materials and Methods. Each value represents the mean ± s.d. of three determinations. ***P < 0.005.
DISCUSSION
The present study demonstrates that LPC induces IFN-γ secretion in PBMC. LPC is a compound present in the lipid moiety of oxidized LDL, but is also induced enzymatically by phospholipase A2, which we recently described in atherosclerotic lesions and arteries [26]. The effects of LPC varied between individuals. LPC containing both unsaturated and saturated fatty acids, and LPC:16, with only saturated fatty acids, could both induce enhanced IFN-γ secretion, indicating that oxidation is not a prerequisite for the effects described here.
We recently reported that oxLDL has the capacity to induce enhanced antibody formation [10], and we here demonstrate that this was the case also with LPC. We have recently detected antibodies to LPC and shown that LPC is an important antigen for antibodies binding to oxLDL [21], and their possible role in cardiovascular disease is presently under investigation.
Recently, we found that oxLDL induces IFN-γ secretion by a PAF receptor-dependent mechanism [12]. Also, LPC-induced IFN-γ secretion and antibody formation were inhibited by a specific PAF receptor antagonist. Taken together, the results indicate that LPC is one mediator of immune activation by oxLDL. LPC in itself does not bind to the PAF receptor, though it has structural similarities with PAF [27,28]. One explanation of our finding could be that LPC undergoes enzymatic acetylation, becoming a PAF-like lipid [27,28]. Recently, LPC was shown to transduce Ca2+ via the PAF receptor in macrophages [29], a finding which is in accordance with our findings presented here.
Since LPC is produced by secretory phospholipase A2, from phosphotidylcholine, oxidation per se may therefore not be a prerequisite for proinflammatory effects of LDL, but instead, this kind of enzymatic modification involving production of LPC may be of importance for the immune activation in the early phases of atherosclerosis and other inflammatory diseases [26].
IFN-γ may be produced both by T cells and natural killer (NK) cells. The observed immunostimulatory properties of LPC may be related both to unspecific effects, or depend on activation by means of a conventional antigen presented on antigen-presenting cells. It is also possible that T cells already activated by other antigens are further stimulated by oxLDL and LPC. This would be compatible with recent findings indicating an IL-12-inducing capacity of oxLDL [30]. However, the different possible pathways are not mutually exclusive.
Antibodies to cardiolipin recognize β2-glycoprotein I, in complex with a lipid, and these antibodies have been shown to cross-react with oxidized LDL [31]. Recently, antibodies to cardiolipin were shown to be directed against epitopes of oxidized phospholipids [32]. However, it is not known if LPC also may form immunogenic lipopeptide complexes.
Stressed macrophages express heat shock proteins (hsp) which are recognized by T cells [33]. One possibility is that immunogenic hsp such as hsp65, induced by oxLDL [34], may stimulate specific T lymphocytes. In line with this possibility is also recent findings that antibody titres to hsp65 are enhanced in cardiovascular disease [35,36].
Oxysterols are produced during oxidation of LDL and in contrast to LPC they had no significant stimulatory effects, but instead tended to be inhibitory. Oxysterols also have cytotoxic properties [37]. Other factors in the lipid moiety tested, including arachidonic acid and some of its oxidized derivatives, had no effect on IFN-γ secretion.
Using an assay where peripheral blood is diluted with medium, with no prior preparation of PBMC, LPC, like oxLDL [12], induced TNF-α secretion. The main advantage of this assay is that stress is avoided during preparation of monocytes [38].
TNF-α is a cytokine with multiple proinflammatory properties, including induction of enhanced adhesion molecules on endothelial cells and potentiation of immune reactions. Recently, TNF-α has been related to enhanced insulin resistance, which occurs in a metabolic syndrome which is associated with atherosclerosis [39]. OxLDL-stimulated PBMC enhance the expression of adhesion molecules on endothelial cells [40], and TNF-α, induced by LPC, may be responsible for this effect. LPC-induced TNF-α secretion was inhibited by WEB 2170, clearly indicating the importance of the PAF receptor.
Other reports that support the notion of LPC as a proinflammatory factor include the finding that LPC potentates protein kinase C-mediated T cell activation [41] and is chemotactic for T lymphocytes [19]. Taking all data together, it is possible that LPC, produced during oxidation or enzymatic modification of LDL in the artery wall, may attract and promote activation of immune-competent cells and thus participate in inflammatory diseases, including atherosclerosis.
Acknowledgments
This work was supported by Swedish Medical Research Council (B95-19X-11244-01, 03X-07135), King Gustaf V80th Birthday Fund, Lars Hiertas Minne, the Swedish Society of Medicine, The Swedish Heart and Lung foundation, The Swedish Rheumatism Association and Hagbergs Fund.
REFERENCES
- 1.Quinn MT, Parthasarathy S, Fong GL, Steinberg D. Oxidatively modified low density lipoprotein: a potential role in recruitment and retention of monocytes/macrophages during atherogenesis. Proc Natl Acad Sci USA. 1987;84:2995–8. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Eng J Med. 1989;320:915–24. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 3.Frostegård J, Haegerstrand A, Gidlund M, Nilsson J. Biologically modified low density lipoprotein increases the adhesive properties of vascular endothelial cells. Atherosclerosis. 1991;90:119–26. doi: 10.1016/0021-9150(91)90106-d. [DOI] [PubMed] [Google Scholar]
- 4.Frostegård J, Nilsson J, Haegerstrand A, Hamsten A, Wigzell H, Gidlund M. Oxidized low-density lipoprotein induces differentiation and adhesion of human monocytes and the monocytic cell line U937. Proc Natl Acad Sci USA. 1990;87:904–8. doi: 10.1073/pnas.87.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uyemura K, Demer LL, Castle SC, et al. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97:2130–8. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palinski W, Rosenfeld ME, Ylä-Herttuala S, et al. Low density lipoprotein undergoes modification in vivo. Proc Natl Acad Sci USA. 1989;86:1372–6. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salonen JT, Yla-Herttuala S, Yamamoto R, et al. Autoantibody against oxidized LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–7. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 8.Frostegård J, Wu R, Giscombe R, Holm G, Lefvert AK, Nilsson J. Induction of T cell activation by oxidized low density lipoprotein. Arteriosclerosis Thrombosis. 1992;12:461–7. doi: 10.1161/01.atv.12.4.461. [DOI] [PubMed] [Google Scholar]
- 9.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1995;92:3893–7. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YH, Rönnelid J, Frostegård J. Oxidized LDL induces enhanced antibody formation and MHC class II dependent IFN-gamma production in lymphocytes from healthy individuals. Arteriosclerosis, Thromb Vasc Biol. 1995;15:1577–83. doi: 10.1161/01.atv.15.10.1577. [DOI] [PubMed] [Google Scholar]
- 11.Watson AD, Navab M, Hama SY, et al. Effect of platelet activating factor-acetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J Clin Invest. 1995;95:774–82. doi: 10.1172/JCI117726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frostegård J, Huang YH, Rönnelid J, Schäfer-Elinder L. PAF and oxidized LDL induce immune activation by a common mechanism. Arteriosclerosis, Thrombosis Vasc Biol. 1997;17:963–8. doi: 10.1161/01.atv.17.5.963. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeldt ME, Palinski W, Ylä-Herttuala S, Butler S, Parthasarathy S, Witztum JL. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990;10:349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- 14.Björkhem I, Henriksson-Freyschuss A, Breuer O, Diczfalusy U, Berglund L, Henriksson P. The antioxidant butylated hydroxytoluene protects against atherosclerosis. Arteriosclerosis Thrombosis. 1991;11:15–22. doi: 10.1161/01.atv.11.1.15. [DOI] [PubMed] [Google Scholar]
- 15.Esterbauer H, Jürgens G, Quehenberger O, Koller E. Autooxidation of human low density lipoprotein: loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J Lipid Res. 1987;28:495–509. [PubMed] [Google Scholar]
- 16.Brown MS, Kovanen P, Goldstein J. Regulation of plasma cholesterol by lipoprotein receptors. Sci. 1981;212:628–35. doi: 10.1126/science.6261329. [DOI] [PubMed] [Google Scholar]
- 17.Brown MS, Basu SK, Falck JR, Ho YK, Goldstein JL. The scavenger cell pathway for lipoprotein degradation. Specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J Supramol Struct. 1980;13:67–81. doi: 10.1002/jss.400130107. [DOI] [PubMed] [Google Scholar]
- 18.Quinn MT, Parthasarathy S, Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci USA. 1988;85:2805–9. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMurray HF, Parthasarathy S, Steinberg D. Oxidatively modified low density lipoprotein is a chemoattractant for human T lymphocytes. J Clin Invest. 1993;92:1004–8. doi: 10.1172/JCI116605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kume N, Cybulsky MI, Gimbrone MA. Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992;90:1138–44. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu R, Huang YH, Schäfer-Elinder L, Frostegård J. Lysophosphatidylcholine is involved in the antigenicity of oxLDL. Arteriosclerosis Thromb Vasc Biol. 1998;18:626–30. doi: 10.1161/01.atv.18.4.626. [DOI] [PubMed] [Google Scholar]
- 22.Heuer H, Birke F, Brandt K, Muacevic G, Weber KH. Biological characterization of the enantiomeric hetrazepines of the PAF-antagonist WEB 2170. Prostaglandins. 1988;35:847. [Google Scholar]
- 23.Czerkinsky C, Andersson G, Ekre HP, Nilsson LA, Klareskog L, Ouchterlony Ö. Reverse ELISPOT assay for colonel analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988;110:29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- 24.Rönnelid J, Huang YH, Norrlander T, Rogberg S, Nilsson B, Gustafsson R, Klareskog L. Short term kinetics of the humoral anti-C1q response in SLE using the ELISPOT method: fast decline in production in response to steroids. Scand J Imunol. 1994;40:243–50. doi: 10.1111/j.1365-3083.1994.tb03457.x. [DOI] [PubMed] [Google Scholar]
- 25.Czerkinsky C, Nilsson L, Nygren H, Ouchterlony Ö, Tarkowski A. A solid-phase enzyme linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–21. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 26.Schäfer-Elinder L, Hedin U, Dumitrescu A, Larsson P, Frostegård J, Claesson HE. Presence of different isoforms of phospholipase A2 in tissue slices from atherosclerotic carotid plaque. Arteriosclerosis Thromb Vasc Biol. 1997;17:2257–62. doi: 10.1161/01.atv.17.10.2257. [DOI] [PubMed] [Google Scholar]
- 27.Tokumura A. A family of phospholipid autacoids: occurrence, metabolism and bioactions. Prog Lipid Res. 1995;34:151–84. doi: 10.1016/0163-7827(95)00001-g. [DOI] [PubMed] [Google Scholar]
- 28.Sparrow CP, Parthasarathy S, Steinberg D. Enzymatic modification of low density lipoprotein by purified lipoxygenase plus phospholipase A2 mimics cell-mediated oxidative modification. J Lipid Res. 1988;29:745–53. [PubMed] [Google Scholar]
- 29.Ogita T, Tanaka Y, Nakaoka T, Matsuoka R, Kira Y, Nakamura M, Shimizu T, Fujita T. Lysophosphatidylcholine transduces Ca2+ signiling via the platelet-activating factor receptor in macrophages. Am J Physiol. 1997;272:17–24. doi: 10.1152/ajpheart.1997.272.1.H17. [DOI] [PubMed] [Google Scholar]
- 30.Trinchieri G, Gerosa F. Immunoregulation by interleukin-12. J Leukocyte Biol. 1996;59:505–11. doi: 10.1002/jlb.59.4.505. [DOI] [PubMed] [Google Scholar]
- 31.Vaarala O, Alfthan G, Jauhiainen M, Leirisalo-Repo M, Aho K, Palosuo T. Crossreaction between antibodies to oxidised low-density lipoprotein and to cardiolipin in systemic lupus erythematosus. Lancet. 1993;341:923–5. doi: 10.1016/0140-6736(93)91213-6. [DOI] [PubMed] [Google Scholar]
- 32.Hörkkö S, Miller E, Dudl E, et al. Antiphospholipid antibodies are directed against epitopes of oxidized phospholipids. J Clin Inv. 1996;98:815–25. doi: 10.1172/JCI118854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koga T, Wand-Wurttenberger A, De Bruyn J, Munk ME, Schoel B, Kaufmann SH. T cells against a bacterial heat shock protein recognize stressed macrophages. Sci. 1989;245:1112–5. doi: 10.1126/science.2788923. [DOI] [PubMed] [Google Scholar]
- 34.Frostegård J, Kjellman B, Gidlund M, Andersson B, Jindal S, Kiessling R. Induction of heat shock protein in monocytic cells by oxidized low density lipoprotein. Atherosclerosis. 1996;121:93–103. doi: 10.1016/0021-9150(95)05706-4. [DOI] [PubMed] [Google Scholar]
- 35.Frostegård J, Lemne C, Andersson B, Kiessling R, de Faire U. Association of serum antibodies to heat shock protein 65 with borderline hypertension. Hypertension. 1997;29:40–44. doi: 10.1161/01.hyp.29.1.40. [DOI] [PubMed] [Google Scholar]
- 36.Xu Q, Willeit J, Marosi M, et al. Association of serum antibodies to heat shock protein 65 with carotid atherosclerosis. Lancet. 1993;341:255–9. doi: 10.1016/0140-6736(93)92613-x. [DOI] [PubMed] [Google Scholar]
- 37.Hughes H, Mathews B, Lenz ML, Guyton JR. Cytotoxicity of oxidized LDL to porcine aortic smooth muscle cells is associated with the oxysterols 7-ketocholesterol and 7-hydroxycholesterol. Arterioscler Thromb. 1994;14:1177–85. doi: 10.1161/01.atv.14.7.1177. [DOI] [PubMed] [Google Scholar]
- 38.Lundahl J, Hallden G, Hallgren M, Skold CM, Hed J. Altered expression of CD11b/CD18 and CD62L on human monocytes after cell preparation procedures. J Immunol Methods. 1995;180:93–100. doi: 10.1016/0022-1759(94)00303-e. [DOI] [PubMed] [Google Scholar]
- 39.Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci USA. 1994;91:4854–8. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frostegård J, Wu R, Haegerstrand A, Patarroyo M, Lefvert AK, Nilsson J. Mononuclear leucocytes exposed to oxidized low density lipoprotein secrete a factor that stimulates endothelial expression of adhesion molecules. Atherosclerosis. 1993;103:213–9. doi: 10.1016/0021-9150(93)90264-u. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki Y, Asaoka Y, Nishizuka Y. Potentiation of diacylglycerol-induced activation of protein kinase C by lysophospholipids. Subspecies difference. FEBS Letters. 1993;320:47–51. doi: 10.1016/0014-5793(93)81655-j. [DOI] [PubMed] [Google Scholar]


