Abstract
Serological rebounds occur frequently in patients with congenital toxoplasmosis, but remain poorly understood. A link between Th1 and Th2 cytokines and the pathophysiology of infectious diseases has been reported. Production of interferon-gamma (IFN-γ) and IL-4 in supernatants of whole blood after in vitro specific Toxoplasma gondii stimulation and serum-specific IgE levels were studied in 31 congenitally infected children. IFN-γ was produced at higher levels by lymphocytes from children with stable congenital toxoplasmosis (n = 18) than from children showing serological rebound (n = 13) (P < 0.04). Conversely, supernatants from children with serological rebound showed higher levels of IL-4 than those from children with stable congenital toxoplasmosis (P < 0.03). The polarized Th2 response was confirmed by a greater (IL-4:IFN-γ) × 100 ratio (P < 0.0001) and production of T. gondii-specific IgE in six out of 13 children showing serological rebound. These results suggest a role of Th2 cytokines in destabilization of congenital toxoplasmosis and perhaps in local reactivation of the parasite.
Keywords: congenital toxoplasmosis, serological rebound, IgE, IL-4, interferon-gamma
INTRODUCTION
While generally asymptomatic in acquired forms, human infection with the parasite Toxoplasma gondii causes severe mortality and morbidity in patients with immunodeficiency or in congenitally infected children [1]. In particular, congenital toxoplasmosis (CT) is a problematic disease for the clinician because of its unexpected serological and clinical evolution. Significant rises in T. gondii-specific antibody titres (serological rebound) frequently occur long after initial infection, but are still poorly understood [2,3]. We recently demonstrated that B cells spontaneously secreting T. gondii IgG antibodies are present in the peripheral circulation of children showing serological rebounds, suggesting a reactivation of the parasite [4].
It is now widely accepted that T helper (Th) cell responses in mouse models are polarized to Th1 or Th2 type according to the cytokines synthesized which govern the pathophysiology of infectious diseases [5,6]. Th1 cells secrete interferon-gama (IFN-γ), IL-2 and tumour necrosis factor-beta (TNF-β), and are involved in protection against intracellular parasites. Th2 cells produce IL-4, IL-5, IL-10 and IL-13, which favour exacerbation of the disease [7]. Resistance against T. gondii requires cellular immunity, and the role of Th1 and Th2 cytokines has been investigated mainly in murine models of toxoplasmosis [8–10]. In particular, IFN-γ can induce the formation of cysts containing slowly dividing bradyzoites, which persist and normally remain quiescent for life [11]. In humans, few data are available and characterization of Th1 and Th2 profiles are based upon results from mice or experiments based on the use of T cell clones [12–14]. Therefore, the existence of an imbalance between Th1 and Th2 cytokines in the various forms of human toxoplasmosis remains hypothetical [15].
To understand better the role of cytokines in the pathophysiology of human CT, we investigated Th1 and Th2 profiles of lymphocytes from congenitally infected children. Using a recently described method for simple whole blood culture [16], we measured IFN-γ and IL-4 secreted in supernatants after in vitro stimulation with T. gondii. Our results suggest a predominant Th2 profile in congenitally infected children with serological rebound.
SUBJECTS AND METHODS
Subjects
All congenitally infected children are routinely followed up serologically and clinically at our Parasitology Department at yearly intervals unless an abrupt rise in serum antibodies occurs, when they are seen at 3-monthly intervals. Children seen in the interval from May 1997 to June 1998 were tested on their first presentation for cytokine profile as described below. Specific T. gondii IgG and IgM were evaluated by indirect immunofluorescence (Biomérieux, Marcy l'Etoile, France). Children whose IgG titres remained stable and < 60 U over the preceding year were classified as stable CT. Children with a clear increase in IgG titres to T. gondii (> 200 U) in the preceding 12 months were classified as CT with serological rebound.
Adults with serological evidence of recent or chronic acquired toxoplasmosis were included as a positive control group. Children free of T. gondii infection but born to mothers who had seroconverted during pregnancy were included as a negative control group.
Antigen preparation
Soluble T. gondii antigen was prepared by infection of murine WEHI 164 cells (ATCC CRL 1751), at three tachyzoites/cell, with T. gondii strain RH from the peritoneal cavities of 24-h-infected OF1 mice (Iffa Credo, Saint Germain sur l'Arbresle, France). At 2 days the tachyzoites were harvested, washed, adjusted to 106/ml in PBS (Biomérieux), and disrupted by five freeze–thaw cycles. The suspension was clarified by centrifugation at 2500 g for 15 min and filtered through 0.2-μm membranes. Control culture supernatant medium was collected from uninfected WEHI 164 cells.
Whole blood cultures
A sample of peripheral blood was collected once from each child by venipuncture into Vacutainer tubes containing lithium heparin (Becton Dickinson, Meylan, France). Blood was processed after storage for not more than 8 h at room temperature. Phenotype of T cells, specific cellular responses and cytokine quantities were evaluated on whole blood cultures as described previously [16]. Briefly, duplicate samples of 50 μl of whole blood were stimulated with either T. gondii soluble antigen (final concentration 6 μg/ml) or an equal volume of control medium for 7 days at 37°C in 45 × 8.8-mm tubes (Micronic Systems, Lelystad, The Netherlands). Cultures were supplemented at 24 h with 500 μl of RPMI 1640 medium containing 1% l-glutamine, penicillin 10 000 U/ml, streptomycin 10 mg/ml, and amphotericin B 25 mg/ml (Sigma, St Quentin Fallavier, France). On day 7, culture supernatants were collected from each tube, clarified by centrifugation at 8000 g for 15 min and stored at −20°C until determination of cytokine levels. Incubation times for optimal cellular responses and cytokine detection in supernatants had previously been determined by kinetic assays (data not shown).
Phenotype of T cells
After collection of supernatants for cytokine assays, we determined the proportion of CD3+ cells which expressed CD4, in samples stimulated with T. gondii soluble antigen or with control medium, as previously described [16].
Cellular responses
The cellular immune responses of the blood cells were evaluated by flow cytometric detection of CD25 [16], and expressed as the percentage of T cells expressing CD25 above control levels. The threshold of positivity was set at 10.1%.
Quantification of IFN-γ and IL-4 levels in 7-day culture supernatants
IFN-γ levels were determined by ELISA using a standard Duoset kit (Genzyme, Cergy St. Christophe, France) following the manufacturer's recommendations. Supernatants were tested at dilution 1:4 in RPMI 1640 medium supplemented with fetal calf serum (FCS; Sigma). The minimum threshold of this assay was 15 pg/ml.
IL-4 was assayed by the Pelikine compact ELISA kit from CLB (Tebu, Le Perray-en-Yvelines, France). Supernatants were tested undiluted following the manufacturer's recommendations. The lower threshold of this test was 2 pg/ml.
Each supernatant was tested in duplicate in 96-well Maxisorp microtitre plates (Nunc Immuno Plate; Polylabo, Strasbourg, France), and results were expressed as means. After transformation of optical density into pg/ml, specific IFN-γ and IL-4 secretion were measured by subtracting cytokine levels of control medium-stimulated samples from cytokine levels of T. gondii-stimulated samples.
Specific IgE secretion
Toxoplasma gondii-specific IgE in the sera from congenitally infected children was quantified by ELISA immunocapture adapted from a previously reported technique [17], in the same blood sample used for the other assays.
Statistical analysis
Differences in secretion between each group of patients were tested for significance with the Mann–Whitney rank test. P < 0.05 was considered statistically significant.
RESULTS
Study population
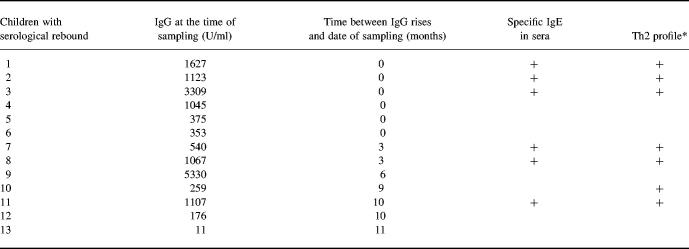
Of the 31 congenitally infected children enrolled in the study, 18 (age 10 ± 3 years (mean ± s.d.)) had stable CT and 13 (age 4 ± 3 years) showed serological rebound. IgG titres at the date of sampling and the length of time elapsed since maximum IgG titres had been observed in the 13 children with serological rebound are shown in Table 1. Specific IgM antibodies were never detected. No new clinical and ophthalmological signs were diagnosed at the date of sampling, nor during a 6-month follow up. Negative and positive control groups consisted of five children free of T. gondii infection (age 1 ± 0 years) and 27 adults (age 32 ± 7 years) with serologic evidence of acquired toxoplasmosis, respectively.
Table 1.
Characterization of the 13 children with serological rebound
At the date of sampling, specific IgG and IgE titres and Th2 cytokine profiles were measured as described in Subjects and Methods.
*Samples with an (IL-4:IFN-γ) × 100 ratio > 2.51 were considered to have a Th2 profile.
Children nos 1–6 showed serological rebound during the May 1997 to June 1998 time interval when cytokine profiles were determined. The other children had rebound from dates preceding the study period.
Phenotype of T cells
In control medium-stimulated samples, percentages of CD4+ T cells were significantly higher in children with serological rebound than in children with stable CT (67.1 ± 10.2% versus 60.1 ± 8%; P = 0.022). After T. gondii-specific stimulation, percentages of CD4+ T cells increased significantly in both children with serological rebound (P = 0.019) and children with stable CT (P < 0.0001).
Cellular responses
CD25 expression on T cells was determined for each group of subjects. All individuals, excepted the five uninfected children, responded positively to T. gondii antigen by strong expression of CD25 (data not shown). CD25+ T cells were exclusively CD4+ in 23 of 31 children, but a minority of CD4− cells were present in the others.
IFN-γ and IL-4 secretion
Supernatants of control medium-stimulated and T. gondii-stimulated cultures from uninfected controls had no detectable levels of IFN-γ or IL-4. Conversely, in acquired toxoplasmosis IFN-γ and IL-4 were secreted after T. gondii stimulation in supernatants of blood cultures from 26 (220–5409 pg/ml) out of 27 individuals and 18 (0.4–10.6 pg/ml) out of 27 individuals, respectively.
IFN-γ was undetectable in supernatants of control medium-stimulated cultures from congenitally infected children. Toxoplasma gondii-driven IFN-γ production was significantly higher in supernatants from children with stable CT than in those of children showing serological rebound (P < 0.04) (Fig. 1a). Low levels of IL-4 were detected in supernatants of control medium-stimulated cultures from almost all children. No significant differences were observed between groups. Toxoplasma gondii-driven IL-4 production was significantly higher in supernatants from children showing serological rebound than from children with stable CT (P < 0.03) (Fig. 1b).
Fig. 1.
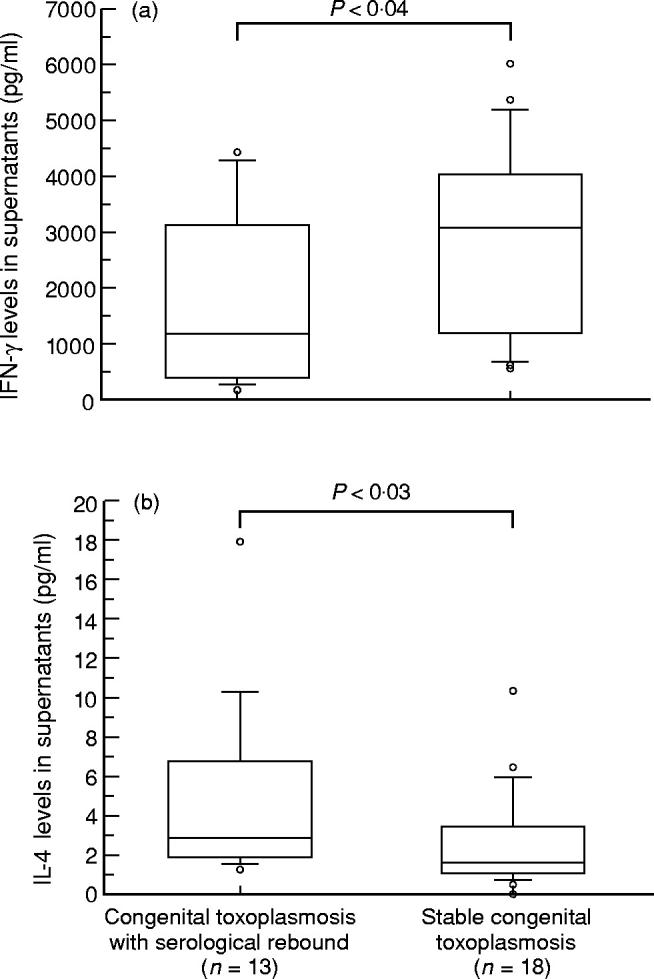
Production of cytokines in 18 children with stable congenital toxoplasmosis (CT) and 13 children showing serological rebound. IFN-γ (a) and IL-4 (b) were measured in supernatants of whole blood after 7 days incubation with Toxoplasma gondii antigen as described in Subjects and Methods. Levels of cytokines above controls were expressed in pg/ml. Boxes represent values between 25th and 75th percentiles and medians; bars indicate 10th and 90th percentiles. Circles outside the bars are extra values.
The cellular response from all patients, expressed as the percentage of CD3+CD25+ cells, correlated with IFN-γ (r = 0.570, P < 0.0001) and IL-4 levels (r = 0.443, P = 0.0004) after T. gondii stimulation. Because IFN-γ can be produced either by CD4+ or by CD8+ activated T cells, we determined the correlation between IFN-γ production in supernatants and the percentages of CD4+CD25+ T cells and found it significantly positive (r = 0.428; P = 0.0007).
IL-4:IFN-γ ratio
The balance between Th1- and Th2-type responses was estimated by the ratio between supernatant IL-4 and IFN-γ (pg/ml), multiplied × 100 to adjust for different levels of production. Values of (IL-4:IFN-γ) × 100 were higher in children showing serological rebound (4.80 ± 6.50) than in children with stable CT (1.02 ± 0.75), with P < 0.0001 (Fig. 2). More than half (7/13) children showing rebound had ratios above 2.51, average stable CT value + 2 s.d., indicating a strong bias to Th2 response. A Th2 profile was also observed in one child with stable CT.
Fig. 2.
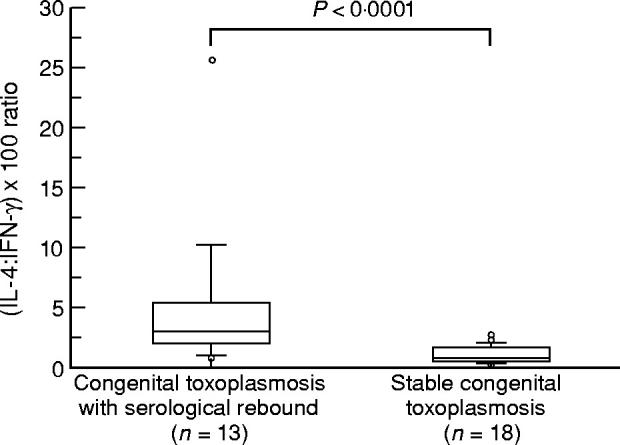
Ratio (IL-4:IFN-γ) × 100 in 18 children with stable congenital toxoplasmosis (CT) and 13 children showing serological rebound. Boxes represent values between 25th and 75th percentiles and medians; bars indicate 10th and 90th percentiles. Measures outside the bars are shown as circles. Statistical analysis (Mann–Whitney U-test) showed significantly higher (IL-4:IFN-γ) × 100 ratio in patients with serological rebound than in patients with stable CT (P < 0.0001).
IgE specific for T. gondii in sera
All six children positive for T. gondii-specific IgE in sera showed serological rebound. No correlation was observed between IgG and IgE titres. There were no clinical differences between IgE+ and IgE− children. We then examined the correlation between the presence of Th2 profile and specific IgE, and the length of time elapsed since rebound in the 13 children (Table 1). The majority (5/7) of children with Th2 profile showed recent serological rebound (≤ 3 months); all had specific IgE in sera. The other two had ancient rebounds, with both specific IgE and persistent high IgG titres in the serum of one of them (child no. 11). Three of six children with serological rebound, but without specific IgE or Th2 profile, had recent rises in IgG titres (nos 4, 5 and 6).
DISCUSSION
Our study is the first attempt to explore and characterize Th1 and Th2 profiles in human toxoplasmosis from ex vivo blood samples without cloning of T cell populations. Measurement of cytokines in whole blood cultures is well established and provides more information about mechanisms governing cellular immunity than use of purified cells [18–20]. By avoiding separation procedures, the technique requires only small amounts of whole blood and so is suitable for studies in children [19,21]. This is a simple and rapid test easily applicable to routine examination
Using sensitive ELISA tests we detected IFN-γ and IL-4 in supernatants of whole blood culture following in vitroT. gondii stimulation. Both cytokines were detected in the positive control group consisting of adults with acquired toxoplasmosis. The positive correlation between CD25+ T cells and levels of IFN-γ and IL-4 in supernatants suggested that T. gondii-responding T cells were responsible in part for cytokine production. Moreover, production of IFN-γ was due in part to the predominant CD4+ subset of responding T cells. We observed opposite cytokine profiles in the two groups of children with CT. Blood cultures from children showing serological rebound produced higher amounts of IL-4 but lower levels of IFN-γ than cultures from children with stable CT. Moreover, a significantly higher (IL-4:IFN-γ) × 100 ratio in children showing serological rebound suggested a switch towards Th2. Th2 cytokines are involved in the generation and maintenance of antibody, particularly IgE [5,22]. In our study, the Th2 profile in seven out of 13 children showing serological rebound was confirmed by the presence of specific IgE in six of them (Table 1). Experimental models of murine toxoplasmosis suggest that a Th1 and Th2 balance is implicated in regulation of T. gondii infection [23]. In particular, IL-4 is responsible for an increased susceptibility to T. gondii in the chronic phase of infection and inhibition of IFN-γ production in vivo [24,25]. Little is known about profiles of cytokine secretion in human toxoplasmosis. In a recent review, Gomez-Marin et al. postulated that the various clinical forms of human toxoplasmosis might be associated with the predominance of one or other pole. Ocular toxoplasmosis (in immunocompetent patients) might be attributed to a Th1 hyperresponse, whereas CT or toxoplasmic encephalitis (in immunodeficient patients) might be characterized by a predominantly Th2 response [15]. Two recent studies carried out on human T. gondii-specific T cell clones demonstrated production of both Th1 and Th2 cytokines after specific stimulation [13,14].
Some hypotheses could explain the Th2 switch observed in children with serological rebound. Lymphocytes from immunocompetent adults with acquired toxoplasmosis probably display a dominantly Th1 profile favouring protection and control of the T. gondii infection. In children with CT, T cell profiles might be unstable, probably because of in utero priming with antigen and tolerization inducing immune deviation [26]. Disturbance of the host–microorganism balance following, for example, bacterial or viral infections and/or drug withdrawal, which has been associated with serological rebounds [3,27], could lead to dominance of Th2 in some children. Such immunological modification would be expected to result in rupture of cysts and/or release of parasite antigen which could consequently reactivate the immune system, producing both specific T. gondii Th2 cells and the specific humoral IgE and IgG response. We recently demonstrated specific circulating antibody-secreting cells in some children with serological rebound, suggesting a parasitic stimulation of the immune system [4]. Sklenar et al. showed that T cell clones produced more IFN-γ when they originated from an asymptomatic T. gondii-infected subject than from a symptomatic subject [28]. The Th2 profile observed in seven out of 13 children with rebound does not indicate clinical repercussions, as no new symptoms appeared at the date of sampling. Secretion of IFN-γ in all cases certainly tempered evolution of the disease and restored the host–parasite balance without any damage. In leprosy and leishmaniasis a Th2 profile accompanied by impairment of IFN-γ leads to critical and severe diseases [29,30]. Presence of serological rebound with low (IL-4:IFN-γ) × 100 ratio and absence of IgE in the other six children could have several explanations. First, IgE are transitory [17] and three children had ancient serological rebound (> 3 months). For the other three children, although the sample tested was contemporaneous with the first routine sample showing increased IgG antibody, the actual date of rebound in the year elapsed since the previous sample is unknown. Second, serological rebound could be the result of a polyclonal activation of cells, and indeed other antibodies may rise concomitantly with anti-T. gondii titres (M. Wallon, personal communication).
The pathophysiology of rebounds remains unknown. They occur in 87% of children with CT, generally without clinical signs [3,27], and they are six times as frequent within 4–6 months after treatment withdrawal (M. Wallon, personal communication). In some cases rebounds are accompanied by the presence of T. gondii-specific circulating B cells [4]. In the present study seven out of 13 rebounds were associated with a Th2 cytokine profile but without clinical signs. Other techniques like detection of circulating T. gondii antigen would be desirable to confirm the existence of a reactivation of the parasite. For the clinician, understanding of serological rebounds raises the issue of management and therapeutic indications in congenitally infected children.
Acknowledgments
We thank Dr J. Bienvenu for technical assistance and advice concerning cytokine detection.
REFERENCES
- 1.Darcy F, Santoro F. Toxoplasmosis. In: Kierszenbaum F, editor. Parasitic infections and the immune system. San Diego: Academic Press Inc; 1994. pp. 163–201. [Google Scholar]
- 2.Koppe JG, Loewer-Sieger DH, de Roever-Bonnet H. Results of 20-year follow-up of congenital toxoplasmosis. Lancet. 1986;i:254–6. doi: 10.1016/s0140-6736(86)90785-3. [DOI] [PubMed] [Google Scholar]
- 3.Fortier B, Coignard-Chatain C, Dao A, et al. Etude des poussées cliniques évolutives et des rebonds sérologiques d'enfants atteints de toxoplasmose congénitale et suivis durant les 2 premières années de vie. Arch Pédiatr. 1997;4:940–6. doi: 10.1016/s0929-693x(97)86088-5. [DOI] [PubMed] [Google Scholar]
- 4.Kahi S, Cozon GJN, Greenland T, et al. Circulating Toxoplasma gondii-specific antibody-secreting cells in patients with congenital toxoplasmosis. Clin Immunol Immunopathol. 1998;89:23–27. doi: 10.1006/clin.1998.4571. [DOI] [PubMed] [Google Scholar]
- 5.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 6.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 7.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 8.Hunter CA, Roberts CW, Alexander J. Kinetics of cytokine mRNA in the brains of mice with progressive toxoplasmic encephalitis. Eur J Immunol. 1992;22:2317–22. doi: 10.1002/eji.1830220921. [DOI] [PubMed] [Google Scholar]
- 9.Burke JM, Roberts CW, Hunter CA, et al. Temporal differences in the expression of mRNA for IL-10 and IFNγ in the brains and spleens of C57BL/10 mice infected with Toxoplasma gondii. Parasite Immunol. 1994;16:305–14. doi: 10.1111/j.1365-3024.1994.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 10.Gazzinelli RT, Wysocka M, Hieny S, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ cells and accompanied by overproduction of IL-12, IFNγ and TNFα. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 11.Bohne W, Heesemann J, Gross U. Induction of bradyzoite specific Toxoplasma gondii antigens in gamma-interferon-treated mouse macrophages. Infect Immun. 1993;61:1141–5. doi: 10.1128/iai.61.3.1141-1145.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saavedra R, Herion P. Human T-cell clones against Toxoplasma gondii: production of interferon-γ, interleukin-2, and strain cross-reactivity. Parasitol Res. 1991;77:379–85. doi: 10.1007/BF00931632. [DOI] [PubMed] [Google Scholar]
- 13.Däubener W, MacKenzie C, Hadding U. Establishment of T-helper type 1- and T-helper type 2-like human Toxoplasma antigen-specific T-cell clones. Immunology. 1995;86:79–84. [PMC free article] [PubMed] [Google Scholar]
- 14.Prigione I, Facchetti P, Ghiotto F, et al. Toxoplasma gondii-specific CD4 + T cell clones from healthy, latently infected humans display a T helper (TH) 0 profile of cytokine secretion. Eur J Immunol. 1995;25:1298–305. doi: 10.1002/eji.1830250525. [DOI] [PubMed] [Google Scholar]
- 15.Gomez Marin JE, Pinon JM, Bonhomme A, et al. Does human toxoplasmosis involve an imbalance in T1/T2 cytokines? Med Hypotheses. 1997;48:161–9. doi: 10.1016/s0306-9877(97)90283-8. [DOI] [PubMed] [Google Scholar]
- 16.Kahi S, Cozon GJN, Greenland T, et al. A rapid flow cytometric method to explore cellular immunity against Toxoplasma gondii in humans. Clin Diagn Lab Immunol. 1998;5:745–8. doi: 10.1128/cdli.5.6.745-748.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinon JM, Toubas D, Marx C, et al. Detection of specific immunoglobulin E in patients with toxoplasmosis. J Clin Microbiol. 1990;28:1739–43. doi: 10.1128/jcm.28.8.1739-1743.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyte M. In vitro production of interleukin-2 by lymphocytes in whole blood and isolated culture. J Clin Lab Immunol. 1988;26:189–93. [PubMed] [Google Scholar]
- 19.Elsässer-Beile U, Von Kleist S, Gallati H. Evaluation of a test system for measuring cytokine production in human whole blood cell cultures. J Immunol Methods. 1991;139:191–5. doi: 10.1016/0022-1759(91)90188-l. [DOI] [PubMed] [Google Scholar]
- 20.Petrovsky N, Harrison LC. Cytokine-based human whole blood assay for the detection of antigen-reactive T cells. J Immunol Methods. 1995;186:37–46. doi: 10.1016/0022-1759(95)00127-v. [DOI] [PubMed] [Google Scholar]
- 21.Weir RE, Morgan AR, Britton WJ, et al. Development of a whole blood assay to measure T cell responses to leprosy: a new tool for immuno-epidemiological field studies of leprosy immunity. J Immunol Methods. 1994;176:93–101. doi: 10.1016/0022-1759(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 22.Pene J, Rousset F, Briere F, et al. IgE production by normal human lymphocytes is induced by IL-4 and suppressed by interferons and prostaglandin E2. Proc Natl Acad Sci USA. 1988;85:6880–4. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bessieres MH, Swierczynski B, Cassaing S, et al. Role of IFN-γ, TNF-α, IL-4 and IL-10 in the regulation of experimental Toxoplasma gondii infection. J Eukaryot Microbiol. 1997;44:87S. doi: 10.1111/j.1550-7408.1997.tb05800.x. [DOI] [PubMed] [Google Scholar]
- 24.Villard O, Candolfi E, Despringre JL, et al. Protective effect of low doses of an anti-IL-4 monoclonal antibody in a murine model of acute toxoplasmosis. Parasite Immunol. 1995;17:233–6. doi: 10.1111/j.1365-3024.1995.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 25.Roberts CW, Ferguson DJP, Jebbari H, et al. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect Immun. 1996;64:897–904. doi: 10.1128/iai.64.3.897-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271:1728–30. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 27.Villena I, Aubert D, Leroux B, et al. Pyrimethamine-sulfadoxine treatment of congenital toxoplasmosis: follow-up of 78 cases between 1980 and 1997. Scand J Infect Dis. 1998;30:295–300. doi: 10.1080/00365549850160963. [DOI] [PubMed] [Google Scholar]
- 28.Sklenar I, Jones TC, Alkan S, et al. Association of symptomatic human infection with Toxoplasma gondii with imbalance of monocytes and antigen-specific T cell subsets. J Infect Dis. 1986;153:315–24. doi: 10.1093/infdis/153.2.315. [DOI] [PubMed] [Google Scholar]
- 29.Misra N, Murtaza A, Walker B, et al. Cytokine profile of circulating T cells of leprosy patients reflects both indiscriminate and polarized T-helper subsets: T-helper phenotype is stable and uninfluenced by related antigens of Mycobacterium leprae. Immunology. 1995;86:97–103. [PMC free article] [PubMed] [Google Scholar]
- 30.Ribeiro-De-Jesus A, Almeida RP, Lessa H, et al. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res. 1998;31:143–8. doi: 10.1590/s0100-879x1998000100020. [DOI] [PubMed] [Google Scholar]