Abstract
The Fas and Fas ligand (Fas/FasL) pathways may play a central role in cytotoxicity or immunoregulation in liver transplantation. Here, in an attempt to examine the role of Fas/FasL on drug-free tolerance, we measured mRNA levels of Fas/FasL in livers by reverse transcriptase-polymerase chain reaction (RT-PCR), and also protein levels of Fas/FasL in livers by immunohistochemistry and in serum by dot blot assay. PVG recipients bearing DA livers showed serious rejection between post-operative (POD) days 7 and 14, but this rejection was naturally overcome without any immunosuppression. Fas gene and protein products were expressed on almost every cell in livers taken from naive rats, and at any time point in both syngeneic and allogeneic orthotopic liver transplantation (OLT) rats. In contrast, FasL mRNA in DA livers was detectable at POD 2, peaked at POD 14, and declined at POD 63 in allogeneic OLT (DA-PVG). Although the FasL gene was detectable in isografts at POD 14, its expression was much lower than in allografts. The time course and localization of FasL expression indicated that the expression of FasL gradually switched from infiltrating cells to hepatocytes when the rejection was naturally overcome and tolerance was induced in this OLT model. Soluble Fas could constitutively be detected at any time point in the serum of the tolerogenic OLT (DA-PVG) rats and was not diminished during the rejection phase. Soluble FasL peaked at POD 14 in allogeneic OLT, while sFasL was significantly lower in the serum of normal and syngeneic OLT rats. These findings suggest that the Fas and FasL pathways, including soluble forms, may contribute to the control of the immune response in this drug-free tolerance OLT model.
Keywords: Fas, Fas ligand, liver transplantation, rat, tolerance
INTRODUCTION
The immunobiology of liver transplantation has been the subject of much scrutiny since it was recognized that it was possible to perform grafts between unrelated individuals and induce donor-specific tolerance in the absence of extra immunosuppressive treatments. This liver-induced tolerance can be observed in both pig and rat orthotopic liver transplantation (OLT) models [1,2]. In certain combinations of donor and recipient strains, such as DA (MHC haplotype RT1a) and PVG (RT1c), the initial rejection reaction against the liver is overcome without any immunosuppressive treatment [3]. However, other DA organs transplanted alone into PVG recipients are promptly rejected [4]. Numerous investigations have demonstrated a number of possible mechanisms as to why rejection is naturally overcome in this tolerogenic OLT model [5–10]. Soluble MHC class I antigens, which can block the action of cytotoxic T cells, are likely candidates to be involved in liver-specific immunity because these antigens are detectable in serum after OLT and persist for the life of the graft [11]. Soluble MHC class I antigen can not be detected when the donor liver allograft is replaced with a syngeneic graft, suggesting that soluble MHC class I molecules are produced by the transplanted liver [12]. However, the administration of purified soluble class I antigens into a heterotopic heart transplantation (HHT) model showed only a temporary delay in rejection rather than permanent graft acceptance [13]. Alternatively, CD8+ T cells activated by donor class I antigen may be trapped in the liver and eliminated by receiving a signal to undergo apoptosis in the transplanted liver graft [14,15]. There is recent experimental evidence that manipulation of the Fas/Fas ligand (FasL) system might provide this type of apoptosis signal to induce allospecific immunosuppression [16–18].
Fas (APO-1/CD95) is a 45-kD type I membrane protein and a member of the tumour necrosis factor (TNF) receptor family, which includes CD40, nerve growth factor (NGF) receptor and lymphotoxin [19]. Fas is widely expressed by many different cell types with abundant expression in the thymus, liver, heart and kidney [20]. Conversely, FasL is a 40-kD type II membrane protein. FasL is expressed not only on immune-privileged organs such as the testis and eye [16,17], but also on numerous tumour and epithelial cells [21,22]. FasL is expressed on activated cytotoxic T lymphocytes (CTL) and natural killer (NK) cells and has an important role in lymphocyte-mediated cytotoxicity [22]. When CTL or NK cells recognize target cells, they become activated and express FasL, which binds to Fas on the surface of the target cells and induces apoptosis of the target cells [23,24]. Alternately, when the target cells express FasL, CTL or NK cells die via apoptosis. It has been proposed that immune-privileged sites expressing FasL can prevent graft rejection, presumably by inducing apoptotic cell death in Fas-expressing donor-reactive CTL. However, the involvement of the Fas/FasL pathway in liver-induced tolerance has not been examined, despite the fact that other studies have reported activation of Fas and perforin pathways in rat liver allograft rejection. Therefore, our study was performed in an attempt to initially investigate the expression pattern of Fas/FasL genes in donor livers at different time points following tolerogenic OLT (DA-PVG). Second, we investigated the localization of Fas and FasL protein products in liver grafts, together with soluble Fas/FasL levels in serum.
MATERIALS AND METHODS
Orthotopic liver transplantation
Inbred male rats, DA (RT1a) and PVG (RT1c) weighing 200–250 g, were housed at the specific pathogen-free (SPF) animal facility, Chang Gung Memorial Hospital Kaohsiung, and allowed free access to water and standard rat chow. OLT was performed under ether anaesthesia using Kamada's method with some modification [4]. The day on which transplantation was performed was designated as post-operative day 0 (POD 0). DA livers were orthotopically transplanted into PVG recipients (OLT (DA-PVG)). OLT (DA-PVG) rats overcome rejection without any immunosuppressive drugs [2–6]. Liver specimens were collected on various POD from OLT (DA-PVG) rats. Liver specimens from syngeneic OLT (DA-DA or PVG-PVG) and untransplanted animals were used as controls.
RNA isolation and reverse transcriptase-polymerase chain reaction
Samples of livers were frozen quickly in liquid nitrogen and stored at −80°C. Total RNA was extracted using a Tri Reagent isolation kit (Molecular Research Center, Inc., Cincinnati, OH), according to the manufacturer's instructions, and dissolved in diethylpyrocarbonate-treated water and quantified by spectrophotometer. Reverse transcription of RNA from sample tissues, followed by polymerase chain reaction (PCR), was used to detect Fas and FasL gene expression [18]. cDNA was synthesized from total RNA in approximately 100 ng in 20 μl of reaction mixture using a first strand cDNA kit (Boehringer Mannheim, Mannheim, Germany), including 1× PCR buffer, 5 mm MgCl2, 1 mm dNTP, 50 U RNase inhibitor, 20 U AMV reverse transcriptase, and 1.6 μg oligo(dT)15 as a primer. The mixture was incubated at 25°C for 10 min and then at 42°C for 60 min, 95°C for 5 min, and 4°C for 5 min. Fifty microlitres of PCR reaction mixture contained 1 μl product of the reverse transcription as template, 1× PCR buffer, 1.5 mm MgCl2, 2.5 U Taq polymerase, and 0.1 μm primers. The sequences of the primers used are as follows: Fas primers: sense 5′GCAATGCTTC TCTCTGTGACCACTG′3, anti-sense 5′GCTGTTGTGCTCGATCT CATCG′3; FasL primers: sense 5′ATAGAGCTGTGGCTACCGG TG′3, anti-sense 5′CTCCAGA GATCAAAGCAGTTCC′3; actin primers: sense 5′GCTGGAGA TGATGCTCCAAGAGCTGTC′3, anti-sense 5′CGATCAGCGA TACCTGGG′3. β-actin was amplified as an internal control to compare relative abundance of PCR products. The PCR condition consisted of denaturing at 94°C for 1 min, annealing at 55–58°C (55°C for actin, 58°C for Fas and FasL) for 1 min, and extension at 72°C for 1 min. PCR was performed at 30 cycles for Fas and FasL, and 25 cycles for actin. PCR products were separated by electrophoresis in 2% agarose gels and stained with ethidium bromide. The target bands were analysed densitometrically by using a GS-700 Imaging Densitometer (BioRad, Hercules, CA). All experiments were repeated more than twice.
Immunohistochemistry
All tissues examined in this study were obtained from OLT and untransplanted rats, immediately snap-frozen in liquid nitrogen and stored at −80°C until analysed. Detection of Fas and FasL antigens was performed using rabbit polyclonal IgG antibodies directed against Fas (M-20)/FasL (C-178) (Santa Cruz, CA). The specificity of both antibodies against rat was tested by using rat-derived recombinant FasL (amino acid sequences 100–278, cat. no. sc-4240WB; Santa Cruz) and Fas protein that we purified from serum of PVG rats using an anti-Fas antibody, M-20, affinity column. Cryosections (6–8 μm thick) were fixed in ice-cold acetone at 4°C for 10 min. After fixation, the specimens were washed three times with PBS and were blocked by blocking buffer (10% fetal calf serum (FCS) in PBS) for 30 min at room temperature. After discarding the blocking buffer, sections were incubated at room temperature for 60 min with polyclonal antibody to Fas and FasL diluted 10 μg/ml in blocking buffer. Sections were then rinsed in three changes of PBS for 5 min and subsequently incubated for 45 min in anti-rabbit IgG alkaline phosphatase conjugate (1:3000 dilution; Sigma, St Louis, MO), followed by development with substrate (Sigma) for 10 min in the dark. The slides were then counterstained with eosin and mounted.
Immunofluorescence assays were performed on cryosections as described above. Blocked sections were incubated for 1 h with rabbit antibody against either Fas or FasL, washed with three 5-min changes of PBS and then incubated for 1 h with FITC-conjugated goat anti-rabbit IgG (1:500 dilution). The sections were then subjected to a further three 5-min washes in PBS before mounting in a solution of 90% glycerol/PBS supplemented with 25 mg/ml 1,4 diazabicyclo(2,2,2) octane (DABCO). In the case of dual labelling experiments MoAbs against either CD3 (Serotec, Oxford, UK) or ATP7B (a gift from Dr Nagano, Kumamoto University, Japan) were also used to detect infiltrating leucocytes and hepatocytes, respectively. A rhodamine-conjugated goat anti-mouse (1:500 dilution) was used to detect binding of the MoAbs. Sections were then examined using an Olympus BX50 fluorescence microscope (Tokyo, Japan). The liver tissues were graded with respect to Fas/FasL expression as follows: −, no expression; + 1, sporadic expression in a few cells; + 2, up to 25% positive cells; + 3, 26–50% positive cells; + 4, 51–75% positive cells; + 5, 76–100% positive cells.
Quantification of soluble Fas/FasL in serum
OLT serum was analysed by dot blot assay for the presence of sFas and sFasL. Serum samples (1 μl) were applied to nitrocellulose (NC) paper and blocked using blocking buffer (5% non-fatty milk in TBST (10 mm Tris–HCl pH 8.0, 0.15 mm NaCl, 0.05% Tween 20) for 1 h at room temperature. The NC paper was then incubated with primary antibody (Fas or FasL) for 1 h at room temperature. After three washes in TBST buffer, the NC paper was transferred to a secondary antibody (anti-rabbit IgG alkaline phosphatase conjugate) for 1 h at room temperature. After three washes in PBS, the NC paper was developed with an alkaline phosphatase substrate (Sigma) for 10 min in the dark. Several concentrations of rat recombinant FasL and our purified Fas as described above were used as standards. The target dots were analysed and quantified densitometrically using an GS-700 Imaging Densitometer (BioRad). All experiments were repeated more than twice.
RESULTS
Analysis of Fas and FasL mRNA levels in tolerogenic rat liver
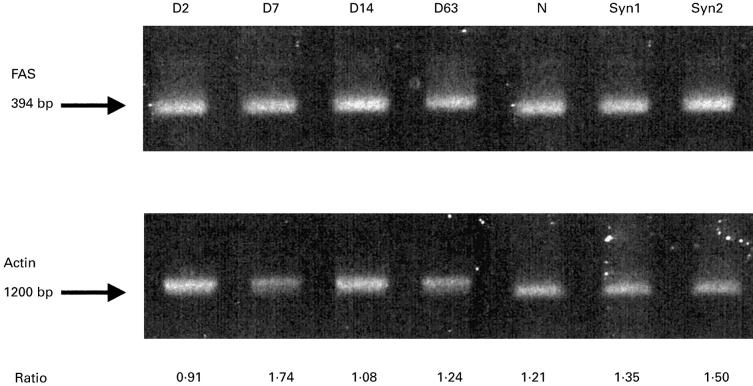
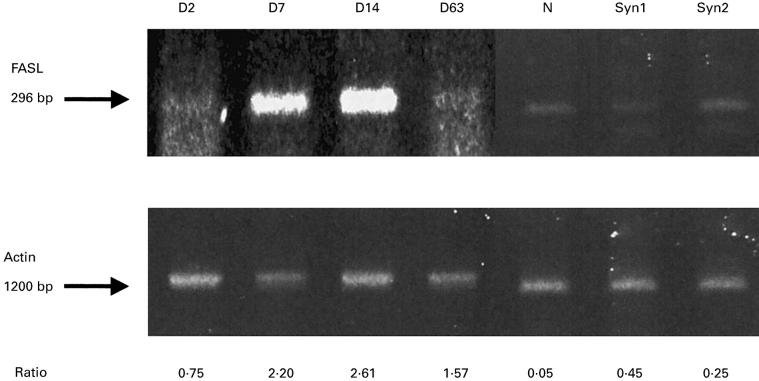
We analysed the RT-PCR of Fas/FasL in liver allografts (DA-PVG), isografts (DA-DA or PVG-PVG), and normal livers (Figs 1 and 2). Fas was constitutively expressed by normal livers, liver isografts and allografts (Fig. 1). In contrast, FasL expression appeared to reflect immunological events caused by the allograft (Fig. 2). In the OLT (DA-PVG) model, FasL in DA livers was detectable at POD 2, peaked at POD 14 when the most serious rejection is observed in this tolerogenic OLT model. This level declined at POD 63 when tolerance had been established in this model. Although the FasL was slightly detectable in isografts at POD 14, its expression was much lower than in allografts, while FasL in normal livers remained undetectable. In an acute rejector OLT (DA-LEW) model, an increased level of FasL expression in liver allografts was observed at POD 7 (data not shown).
Fig. 1.
Fas mRNA expression in liver allografts (DA-PVG), isografts and normal livers. Reverse transcriptase-polymerase chain reaction (RT-PCR) products of Fas are shown. The relative expression to β-actin is shown at the bottom. D, post-operation day; N, normal liver; Syn1, syngeneic OLT (DA-DA) POD7; Syn2, syngeneic OLT (PVG-PVG) POD14.
Fig. 2.
Fas ligand (FasL) mRNA expression in liver allografts, isografts and normal livers. Reverse transcriptase-polymerase chain reaction (RT-PCR) products of FasL are shown. The relative expression to β-actin is shown at the bottom. D, post-operation day; N, normal liver; Syn1, syngeneic OLT (DA-DA) POD7; Syn2, syngeneic OLT (PVG-PVG) POD14.
Fas and FasL expression in tolerogenic liver
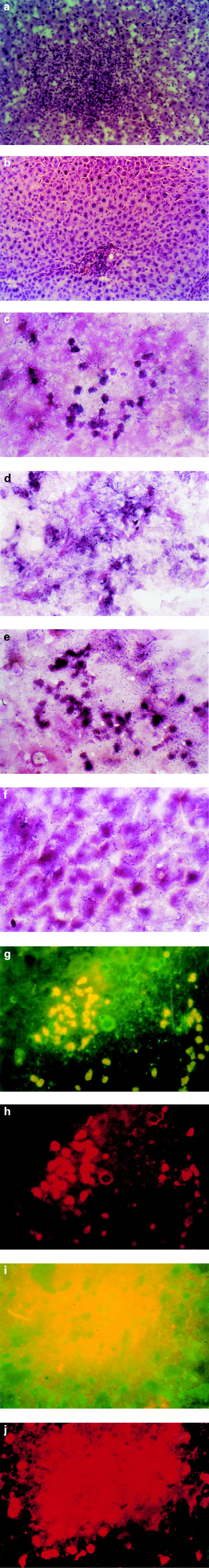
The histology reflected the immunological events following OLT (DA-PVG). At day 14 when the most serious rejection was observed in this tolerogenic OLT (DA-PVG) model, there was dense cell infiltration and scattered focal necrosis of liver cells with mononuclear cells through the sinusoids (Fig. 3a). By 63 days when the rejection was overcome and tolerance was induced, the histological appearance of liver grafts had returned to normal (Fig. 3b). Fas was expressed on almost every cell in livers taken from naive rats, at any time point in syngeneic and allogeneic OLT rats. During the rejection phase of OLT (DA-PVG), Fas was expressed predominantly in infiltrating lymphocytes and hepatocytes at POD 14 in OLT (DA-PVG) livers (Fig. 3c). In the maintenance phase of tolerance in OLT (DA-PVG), Fas was detected in some hepatocytes and vascular endothelium at POD 63 (Fig. 3d). FasL expression was predominantly expressed on infiltrating lymphocytes at POD 14 (Fig. 3e), while Fas L expression decreased on infiltrating lymphocytes but increased on hepatocytes in livers taken at POD 63 (Fig. 3f). The expression sites appeared to be different for Fas and FasL. As shown in Fig. 3, Fas was expressed on the cell surface of hepatocytes (Fig. 3d) and FasL was expressed in the cytoplasmic area of hepatocytes (Fig. 3f). In normal livers and syngeneic OLT liver sections, Fas was constitutively expressed in the hepatocytes while FasL was not expressed in any of our sections (data not shown). The time course and pattern of FasL expression are summarized in Table 1, indicating that the expression of FasL on infiltrating cells gradually decreased after the rejection phase of OLT (DA-PVG) and more hepatocytes subsequently expressed FasL after tolerance was established in this model. We confirmed these findings by double-staining immunofluorescence using CD3 for infiltrating cells and ATP7B for hepatocytes to differentiate the cell types expressing FasL. The number of CD3 and FasL double-positive cells peaked at day 14 (Fig. 3g) and declined at day 63, while a number of ATP7B and FasL double-positive cells could be observed at day 63 (Fig. 3i).
Fig. 3.
Expression of Fas and FasL in hepatocytes and infiltrating leucocytes in the tolerogenic rat orthotopic liver transplantation (OLT) (DA-PVG) model. (a) At day 14 when the most serious rejection was observed in this tolerogenic OLT (DA-PVG) model, there was dense cell infiltration and scattered focal necrosis of liver cells with mononuclear cells through the sinusoids (mag. × 120). (b) By 63 days when the rejection was overcome and tolerance was induced, the above histological appearance of liver grafts returned to normal (mag. × 120). (c) Fas was expressed strongly on infiltrating lymphocytes and weakly on hepatocytes at day 14 (mag. × 240). (d) Fas was expressed on both hepatocytes and endothelium at day 63 (mag. × 240). (e) FasL was expressed on both infiltrating lymphocytes and hepatocytes at day 14 (mag. × 240). (f) FasL was expressed on hepatocytes at day 63 (mag. × 240). (g) CD3+ cells were detected by a rhodamine-conjugated goat anti-mouse antibody and cells expressing FasL by a FITC-conjugated goat anti-rabbit antibody. Cells which appear yellow in colour (i.e. infiltrating lymphocytes) are positive for both CD3 and FasL marker (mag. × 240). (h) Single-positive cells expressing CD3 appeared red in colour by labelling rhodamine (mag. × 240). (i) FasL+ cells were detected by a FITC-conjugated goat anti-rabbit antibody and cells expressing ATP7B by a rhodamine-conjugated goat anti-mouse antibody. Cells which appear yellow in colour (i.e. hepatocytes) are positive for both FasL and ATP7B markers (mag. × 240). (j) Single-positive cells expressing ATP7B appeared red in colour by labelling rhodamine (mag. × 240).
Table 1.
Time course of FasL expression in hepatocytes or infiltrating cells of tolerogenic liver allografts
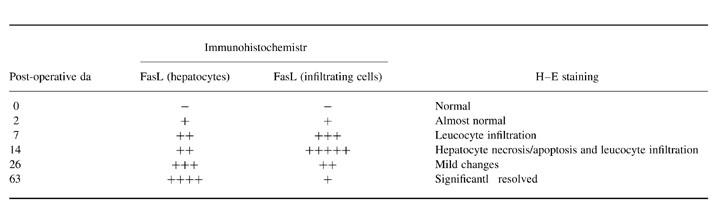
Score for FasL expression: −, no expression; +, sporadic expression in few cells; ++, up to 25% positive cells; +++, 26–50% positive cells; ++++, 51–75% positive cells; +++++, 76–100% positive cells.
Soluble Fas and FasL in serum
Soluble Fas (sFas) was consistently detected in the serum of normal, syngeneic and allogeneic OLT rats (Fig. 4). However, the levels of soluble Fas were not significantly suppressed at the rejection phase following tolerogenic OLT (DA-PVG). Soluble FasL (sFasL) was undetectable or significantly lower in the serum of normal and syngeneic OLT rats compared with sFasL, which peaked at POD 14 in allogeneic OLT (DA-PVG) (Fig. 4).
Fig. 4.
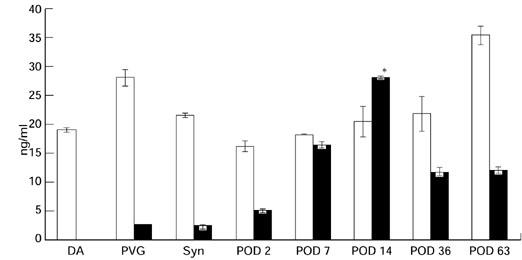
Changes in sFas (□) and sFasL (▪) levels in serum samples from tolerogenic orthotopic liver transplantation (OLT) (DA-PVG) models. Each sample taken from these individual animals was analysed in triplicate for sFas and sFasL by dot blot assay. These data were analysed statistically, by one-way anova and Bonferroni, and found to be significant in POD 14 serum (P < 0.05 versus controls). Syn, Syngenic OLT (PVG-PVG) POD 14.
DISCUSSION
We have demonstrated here that FasL is highly expressed on donor livers, predominantly on infiltrating cells, during the serious rejection phase in tolerogenic OLT (DA-PVG) rats. In an acute rejector OLT (DA-LEW) model, increased levels of FasL were observed on infiltrating donor-reactive T cells, which may increase cytotoxicity against the liver grafts and rapidly turnover by inducing Fas/FasL-mediated apoptosis (data not shown). This scenario also may be applicable to the initial phase when the rejection reaction is observed in tolerogenic OLT (DA-PVG) rats. However, the cells expressing FasL gradually switched from infiltrating cells to donor hepatocytes in the late phase of OLT when the rejection reaction was naturally overcome and tolerance was induced in this combination of OLT. FasL expression was moderately detectable, predominantly in the donor hepatocytes of OLT (DA-PVG) rats, and this reverse expression of FasL from infiltrating cells to hepatocytes may induce an apoptotic signal from the graft to CTL, resulting in allograft tolerance. The replaced Fas/FasL interaction between target cells and CTL has been reported to be important in tumour cells for their immunological evasion [22] or in the eyes and testis for maintaining immune privilege [16,17]. However, other studies have shown that elevated FasL expression resulted in an inflammatory infiltrate and neutrophil-mediated rejection of allografts [25,26]. Thus, it is still too early for us to conclude that the persistent and moderate expression of FasL on transplanted organs may be involved in drug-free tolerance.
Our results appear to show that sFasL may also be involved in establishing drug-free tolerance in tolerogenic OLT (DA-PVG) rats. In this model, the greatest level of sFasL in the serum could be observed at day 14 when the rejection reaction was most serious. There have been some reports concerning sFasL and its function, although it is well known that functional membrane FasL expressed on tumour or grafts also plays an important role in establishing immune evasion of cancer or to achieve an immune-privileged site for allografts. Tanaka et al. reported that sFasL inhibited cytotoxicity of the membrane-bound FasL [27], suggesting that sFasL prevents the killing of healthy bystander cells by cytotoxic T cells. From these reports, increased levels of sFasL in the serum of OLT (DA-PVG) rats during the rejection phase may also play a protective role against Fas/FasL-mediated apoptosis of donor hepatocytes which highly express Fas, as shown in our immunohistochemical study. However, infiltrating cells, including cytotoxic T cells activated against donor antigens, might secrete more sFasL simply for the prevention of self-destruction via desensitizing their Fas receptors rather than the protection of the allograft. Other investigators have suggested that sFasL, as well as membrane-bound FasL, may cause inflammation [28,29]. In our study, FasL expression on infiltrating cells and sFasL peaked at day 14 and declined later, which was consistent with the extent of infiltrating cells in the graft. Therefore, the kinetics of soluble FasL in our results may simply reflect the intensity of inflammation in the allografts.
Fas gene expression had no significant change before and after transplantation in tolerogenic or syngeneic OLT models. There were also no significant changes at the protein level of Fas that were constitutively expressed on almost every cell in the graft and were consistently detectable in the serum of naive and OLT rats. It has been suggested that sFas is produced in the liver by hepatocytes and can specifically inhibit Fas-mediated apoptosis [30–32]. Administration of sFas prevents liver damage in an experimental model of hepatitis [33]. In acute rejector OLT (DA-LEW), the level of sFas was substantially suppressed (data not shown), which is consistent with other clinical evidence that the levels of sFas diminish in patients undergoing liver allograft rejection, in contrast to patients with stable grafts [30]. In our study, sFas was well maintained during the rejection phases and in the maintenance phase of tolerance in tolerogenic OLT (DA-PVG) rats, suggesting that appropriate levels of sFas may play an important role of tolerance induction, although further investigation is required.
The liver is a hostile environment for activated CD8 cells and this might cause peripheral T cell deletion, which may account for unique features of liver allograft tolerance. However, it is still unclear why activated CD8 T cells first accumulate in the liver, and then how these CD8+ cells receive an apoptotic signal at that site. Our results suggest, at least in part, that the Fas and FasL pathway, including soluble Fas and FasL, may induce apoptosis of donor-reactive CD8+ T cells. However, the Fas/FasL pathway in regards to cytotoxicity may not be the only mechanism to be involved in the development of allograft tolerance. Further studies including a link between regulatory CD4+ cells, CD45 and costimulatory factors, and apoptosis, are currently being undertaken.
Acknowledgments
This work was supported by grants from the Chang Gung Medical Research Center Grant-in-Aid (CMRP 783) and the Chao-Long Chen Liver Transplant Foundation.
REFERENCES
- 1.Calne RY, Sells RA, Rena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–6. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 2.Kamada N, Davies HFFS, Roser BJ. Reversal of transplantation immunity by liver grafting. Nature. 1981;292:840–2. doi: 10.1038/292840a0. [DOI] [PubMed] [Google Scholar]
- 3.Kamada N, Brons G, Davies HffS. Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation. 1980;29:429–31. doi: 10.1097/00007890-198005000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Kamada N, Kobayashi E, Goto S. Liver transplantation in the rat. In: Green MK, Mandel TE, editors. Experimental transplantation models in small animals. Chur, Switzerland: Harwood Academic Publishers; 1995. pp. 341–52. [Google Scholar]
- 5.Kamada N, Shinomiya T. Clonal deletion as the mechanism of abrogation of immunological memory following liver grafting in rats. Immunology. 1985;55:85–86. [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshimura S, Goto S, Kamada N. Immunological tolerance induced by liver grafting in the rat: splenic macrophages and T cells mediate distinct phases of immunosuppressive activity. Clin Exp Immunol. 1991;85:121–7. doi: 10.1111/j.1365-2249.1991.tb05692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsdell F, Fowlkes BJ. Clonal deletion versus clonal anergy: the role of the thymus in induction self tolerance. Science. 1990;248:1343–5. doi: 10.1126/science.1972593. [DOI] [PubMed] [Google Scholar]
- 8.Starzl TE, Demetris AJ, Murase N, et al. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J, McCaughan GW, Gallagher ND, et al. Deletion of spontaneous rat liver allograft acceptance by donor irradiation. Transplantation. 1995;60:233–6. doi: 10.1097/00007890-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Chiba S, Goto S, Shimizu Y, et al. The characterisation of reconstituted passenger leukocytes on the induction of tolerance in rat liver transplantation. Transpl Int. 1997;10:350–6. doi: 10.1007/s001470050069. [DOI] [PubMed] [Google Scholar]
- 11.Sumimoto R, Kamada N. Evidence that soluble class I antigen in donor serum induces the suppression of heart allograft rejection in rats. Immunol Letters. 1990;26:81–84. doi: 10.1016/0165-2478(90)90179-t. [DOI] [PubMed] [Google Scholar]
- 12.Goto S, Kamada N, Lord R, Kobayashi E, Enosawa S, Kim Y. Induction of natural chimerism after retransplantation of the liver in rats. Transplantation. 1994;58:1230–5. [PubMed] [Google Scholar]
- 13.Sumimoto R, Kamada N. Specific suppression of allograft rejection by soluble class I antigen and complexes with monoclonal antibody. Transplantation. 1990;26:81–86. doi: 10.1097/00007890-199010000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Nicholas C, Mehal WZ. Strange brew—T cells in the liver. Immunol Today. 1996;17:522–5. doi: 10.1016/s0167-5699(96)80906-6. [DOI] [PubMed] [Google Scholar]
- 15.Crispe N, Flavell R, Leeker M, et al. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity. 1994;1:741–9. doi: 10.1016/s1074-7613(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 16.Bellgrau D, Gold D, Selawry H, et al. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–2. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 17.Yamagami S, Kawashima H, Tsuru T, et al. Role of Fas–Fas ligand interactions in the immunorejection of allogeneic mouse corneal transplants. Transplantation. 1997;64:1107–11. doi: 10.1097/00007890-199710270-00004. [DOI] [PubMed] [Google Scholar]
- 18.Wang JD, Nonomura N, Ichimaru N, et al. Expression of Fas and Fas ligand in renal grafts with acute and chronic rejection in the rat model. Int J Cytokine Res. 1997;17:369–73. doi: 10.1089/jir.1997.17.369. [DOI] [PubMed] [Google Scholar]
- 19.Nagata S, Colstein P. The Fas death factor. Science. 1995;267:1447–56. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 20.Nagata S. Fas ligand and immune evasion. Nature Med. 1996;2:1306. doi: 10.1038/nm1296-1306. [DOI] [PubMed] [Google Scholar]
- 21.Xerri L, Devilard E, Hassoun J, et al. Fas ligand is not only expressed in immune privileged human organs but is also coexpressed with Fas in various epithelial tissues. J Clin Pathol: Mol Pathol. 1997;50:87–91. doi: 10.1136/mp.50.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strand S, Hofmann WJ, Hug H, et al. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand expressing tumour cells—a mechanism of immune evasion? Nature Med. 1996;2:1361. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 23.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–65. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 24.Li XK, Kita Y, Tamura A, et al. Activation of Fas and Perforin pathways in rat liver allograft rejection. Transplant Proc. 1998;30:19–21. doi: 10.1016/s0041-1345(97)01166-4. [DOI] [PubMed] [Google Scholar]
- 25.Allison J, Georgiou HM, Strasser A, et al. Transgenic expression of CD95 ligand on islet β cells induces a granulocytic infiltration but does not confer immune privilege upon islet allografts. Proc Natl Acad Sci USA. 1997;94:3943–7. doi: 10.1073/pnas.94.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang SM, Schneider DB, Lin Z, et al. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nature Med. 1997;3:738–43. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka M, Itai T, Adachi M, et al. Downregulation of Fas ligand by shedding. Nature Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 28.Strasser A, Oconnor L. Fas ligand—caught between Scylla and Charybdis. Nature Med. 1998;4:21–22. doi: 10.1038/nm0198-021. [DOI] [PubMed] [Google Scholar]
- 29.Miwa K, Asano M, Horai R, et al. Caspase 1-independent IL-1β release and inflammation induced by the apoptosis inducer Fas ligand. Nature Med. 1998;4:1287–92. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- 30.Krams SM, Fox CK, Beatty PR, et al. Human hepatocytes produce an isoform of Fas that inhibits apoptosis. Transplantation. 1998;65:713–21. doi: 10.1097/00007890-199803150-00019. [DOI] [PubMed] [Google Scholar]
- 31.Jodo S, Kobayashi S, Nakajima Y, et al. Elevated serum levels of soluble Fas/APO-1 (CD95) in patients with hepatocellular carcinoma. Clin Exp Immunol. 1998;112:166–71. doi: 10.1046/j.1365-2249.1998.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strand S, Hofmann WT, Grambiher A, et al. Hepatic failure and liver cell damage in acute Wilson's disease involve CD95 (APO-1/Fas) mediated apoptosis. Nature Med. 1998;4:588–93. doi: 10.1038/nm0598-588. [DOI] [PubMed] [Google Scholar]
- 33.Adachi M, Suematsu S, Kondo T, et al. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nature Genet. 1995;11:294–9. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]