Abstract
In this study we have compared the immune response of normal human cells cultured in vitro to two virulent strains of Leishmania major(CC1 and LV39), and to an avirulent vaccine strain (dhfr-ts−) made by targeted deletion of the essential gene DHFR-TS. We utilized an in vitro system in which naive T cells from normal human donors were primed with autologous Leishmania-infected macrophages. All three parasites infected macrophages and transformed into amastigotes within the cells. However, whereas LV39 and CC1 replicated in macrophages, dhfr-ts− did not. When peripheral blood lymphocytes (PBL) were stimulated with autologous macrophages infected with any of the three parasites, the lymphocytes produced a type-1-biased cytokine response. Finally, addition of IL-12 during the first stimulation period increased the production of interferon-gamma but decreased IL-5 secretion. On the other hand, anti-IL-12 resulted in the opposite effect.
Keywords: Leishmania major, macrophages, Th1, Th2, IL-12
INTRODUCTION
Protozoan parasites of the genus Leishmania reside within macrophages, causing a spectrum of diseases in humans ranging from mild cutaneous to lethal visceral leishmaniasis. Cell-mediated immune responses are critical to resistance and recovery from leishmaniasis [1].
Cytokines are central elements in the development of an immune response, and in both human and experimental leishmaniasis they have received a great deal of attention. Cure is related to the predominance of a Th1 response, since this leads to the production of interferon-gamma (IFN-γ) and activation of parasite-infected macrophages [2]. In contrast, a Th2 response with the production of IL-4 and IL-10 often results in disease progression [2]. Efforts have focused on understanding the early events that influence the development of Th1 or Th2 cells and the interplay of several cytokines in this phenomenon in order to determine their role in Th development.
In patients infected with Leishmania, it is difficult to estimate the time of initial infection. As a result one cannot study early interactions that occur between the parasite and host. An alternative method for studying these interactions is to use an in vitro system that mimics in vivo infection. Shankar & Titus [3] developed an in vitro system using cells from lymphoid tissues of naive mice and L. major promastigotes and showed that this system mimicked in vivo responses in murine leishmaniasis. Therefore, in this report we developed an in vitro cell priming system (IV) using peripheral blood mononuclear cells (PBMC) from normal donors in an assay in which naive peripheral blood lymphocytes (PBL) were sensitized with autologous Leishmania-infected macrophages.
Recently, Titus et al. [4] tested the safety and vaccination potential of a conditional auxotroph of L. major (dhfr-ts−) produced by targeted deletion of an essential metabolic gene, DHFR-TS (dihydrofolate reductase-thymidylate synthase). It was shown that dhfr-ts− was engulfed by murine macrophages, transformed into amastigotes within macrophages, survived in mice for 2 months and elicited a substantial protective immune response in susceptible BALB/c mice, as indicated by resistance to a subsequent challenge with virulent parasites [4]. In addition, Veras et al. (5) have observed that mice vaccinated with dhfr-ts− were partially protected against a subsequent challenge with L. amazonensis. Therefore, we also studied the capacity of dhfr-ts− to sensitize PBL in our IV system.
MATERIALS AND METHODS
Parasites
Three different types of Leishmania were used in this study: dhfr-ts− (line E-10), an avirulent form of L. major, produced by targeted deletion of an essential metabolic gene DHFR-TS (dihydrofolate reductase-thymidylate synthase [3]); CC1, an attenuated clone of L. major from which dhfr-ts− was derived; and clone 5 of the LV 39 line Rho/SV/59/P, a virulent clone of L. major which was passed through mice every 2 weeks to maintain virulence. When used for in vitro experiments, Leishmania were grown on biphasic NNN medium [6] and the promastigotes were harvested in the stationary phase of growth. Because dhfrt-ts− lack the DFHR-TS gene, it is a conditional auxotroph and thus was cultured in the presence of 10 μg/ml thymidine.
Survival of Leishmania in macrophages in vitro
In order to test the ability of dhfr-ts− to survive in human macrophages in vitro, the cells were adhered to glass coverslips in culture for 7 days and infected with either dhfr-ts−, CC1 or LV39 at a ratio of five parasites to one macrophage. The coverslips were removed at intervals thereafter (24, 48 and 72 h), stained with Diff-Quick (Scientific Products, McGaw, IL) and examined by light microscopy for the presence of intracellular parasites.
Cytokines and monoclonal antibodies
Recombinant human IL-12 (supplied by the Bioanalytical Sciences Department of Genetics Institute, Inc., Cambridge, MA) was used at a concentration of 2.5 μg/ml. Anti-human IL-12 (clone 8.6; PharMingen, San Diego, CA) was used at a concentration of 10 μg/ml.
IV system
The two-step IV assay used in this study is depicted in Fig. 1. From blood collection 1 we obtained the macrophages which served as antigen-presenting cells (APC). Human blood was obtained from healthy individuals at the Student Health Center of Colorado State University. PBMC were obtained from heparinized venous blood by passage over Ficoll–Hypaque gradient [7]. The cells were then washed and resuspended in RPMI 1640 medium, supplemented with 2 mml-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 μg/ml gentamycin and 10% heat-inactivated AB human serum (Pel-Freez; Clynical System, Brown Deer, WI), termed complete medium, at a concentration of 4 × 106 cells/ml. These cells were plated in 24-well plates (Costar, Corning Inc., Corning, NY) and incubated for 2 h at 37°C, 5% CO2. Non-adherent cells were removed from the plates and adherent cells (APC-1, Fig. 1) were cultured (37°C, 5% CO2) in complete medium for 7 days. During the last 24 h of culture, APC-1 were infected with dhfr-ts−, CC1 or LV39 at a parasite:cell ratio of 5:1 (34°C, 5% CO2). Cultures were then washed in order to remove free parasites.
Fig. 1.
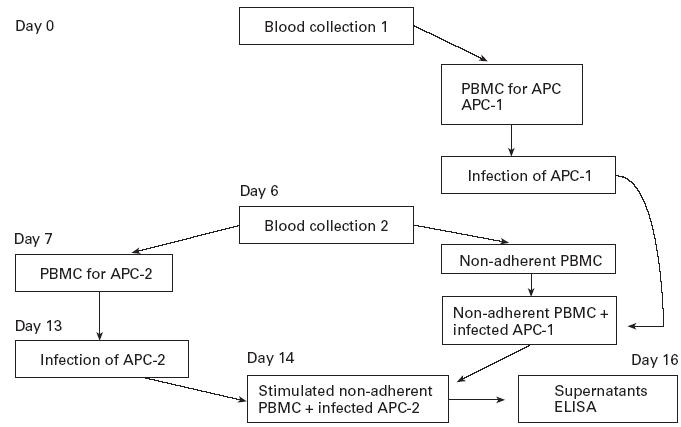
Experimental approach used in the in vitro cell priming system (IV) assays. From blood collection 1 APC-1 were obtained, which were infected after 6 days with the different types of Leishmania used in this study. From blood collection 2 autologous non-adherent peripheral blood mononuclear cells (PBMC) were obtained which were incubated with infected APC-1 for 7 days to generate T cell blasts and APC-2 which were used for restimulation. Twenty-four hours before harvesting the blast cells (i.e. day 13), APC-2 were infected with the different types of Leishmania. On day 14 of the experiment, the blast cells were collected and incubated with infected APC-2 for 48 h. The supernatants were obtained from these cultures and analysed for the levels of IFN-γ and IL-5.
Next, blood was collected from the same volunteer (day 7, Fig. 1) and PBMC were plated at 4 × 106 cells/ml in 24-well plates in order to obtain autologous lymphocytes (non-adherent PBMC) and APC for restimulation (adherent cells, APC-2, Fig. 1). After 2 h of incubation (37°C, 5% CO2), non-adherent PBMC were harvested and resuspended in complete medium at a concentration of 4 × 106 cells/ml. These cells were added to Leishmania-infected APC-1 for 7 days at 37°C, 5% CO2. After this incubation, the blast cells which were 85% CD4+ T cells were harvested and restimulated using autologous Leishmania-infected macrophages (infected APC-2, Fig. 1) for 48 h at 37°C, 5% CO2. The supernatants were collected and kept at −20°C until tested for cytokine content by ELISA.
Cytokine assays
Levels of IFN-γ and IL-5 present in supernatants harvested 48 h after the restimulation were determined using commercial ELISA kits (R&D Systems, Minneapolis, MN).
IL-12 levels were also quantified in 24, 48 or 96 h supernatants harvested from infected and uninfected macrophage cultures using immunoassay kits (R&D Systems) specific for IL-12 p40 and p70 subunits. In some experiments the supernatants obtained after the first period of stimulation (7 days of culture in the presence of infected macrophages and PBL) were harvested and the amount of IFN-γ, IL-5 and IL-12 present in these supernatants was determined by ELISA.
Statistical analysis
Comparisons of the production of cytokines, using different types of Leishmania, were determined using an anova test. Comparisons in the same group (to test the effect of cytokine and anti-cytokine treatment) made use of Student's t-test. All tests were performed using Sigma-Stat software (Microsoft System). Differences were considered significant at P ≤ 0.05.
RESULTS
Inability of dhfr-ts− to replicate in macrophages in vitro
It has been shown that dhfr-ts− was not able to replicate in murine macrophages [3]. Therefore, we tested whether these parasites would replicate in human macrophages in vitro. We infected human macrophages, obtained from normal human donors, with either dhfr-ts−, CC1 or LV39 at a ratio of five parasites to one macrophage. All types of parasites were phagocytized by macrophages in the first 24 h (Fig. 2). Beyond 24 h, virulent Leishmania replicated as amastigotes within macrophages (Fig. 2). In contrast, dhfr-ts− did not replicate. To confirm that the inability of dhfr-ts− to survive in the macrophage was due to the lack of DHFR-TS, we added thymidine (10 μg/ml) to the medium; this restored both survival and replication (Fig. 2).
Fig. 2.
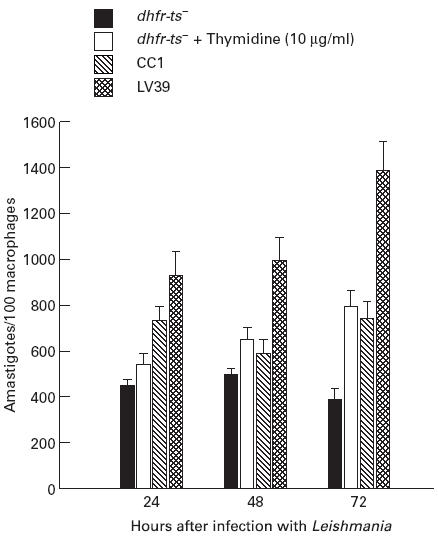
Inability of dhfr-ts− to replicate in human macrophages in vitro. Human macrophages obtained from peripheral blood mononuclear cells (PBMC) were infected with stationary phase Leishmania at a 5:1 ratio (Leishmania:macrophage). At the indicated times, cells were fixed, stained with Diff-Quick and examined by light microscopy for the number of intracellular parasites. One hundred macrophages/donor were counted each time for the number of intracellular amastigotes. Results are shown as the mean ± s.e.m. for three samples in each group.
Cytokine production
Using the IV assay depicted in Fig. 1, the production of IFN-γ and IL-5 was quantified, since these cytokines are considered representative of Th1 and Th2 responses, respectively [1]. In initial experiments we also attempted to measure IL-4 production in the cultures, but IL-4 could not be consistently detected, presumably because of consumption of the cytokine by proliferating cells. Figure 3 shows the concentration of IFN-γ and IL-5 obtained using Leishmania-infected adherent cells (APC) and autologous non-adherent cells. All types of parasites were able to induce the production of IFN-γ and no significant differences were observed between the responses generated using dhfr-ts−, CC1 or L. major (P > 0.05). No significant differences were observed also concerning the production of IL-5, but the PBMC from some donors did not produce this cytokine during the IV assay. Based on these results we conclude that most of the donors (6/10) used in this study secreted high amounts of IFN-γ, but IL-5 production was practically non-detectable (< 10 pg/ml). Thus, cells from these donors produced predominantly type-1 cytokines. However, three donors produced IL-5 in significant amounts in addition to IFN-γ, which is characteristic of a Th0 response. One of the donors was considered a non-secretor, because the amount of IFN-γ and IL-5 produced was very low. It should be noted that the profile of cytokines observed was not altered when the supernatants were harvested after 7 days of culture using Leishmania-infected APC-1 or 48 h after the second stimulation using infected APC-2 (data not shown).
Fig. 3.
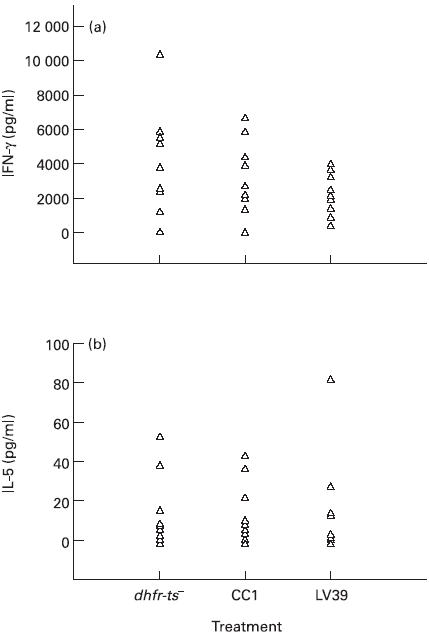
Levels of IFN-γ and IL-5 present in supernatants collected in the in vitro cell priming system (IV) assays. Non-adherent peripheral blood mononuclear cells (PBMC) from normal donors were co-cultured with autologous infected or uninfected macrophages for 7 days, and after this period they were restimulated for 48 h, again employing autologous infected macrophages. Supernatants were collected and cytokine levels determined by ELISAs. (a) Production of IFN-γ (pg/ml). (b) Production of IL-5 (pg/ml). Each triangle represents an individual donor. Concentrations of IFN-γ and IL-5 in cultures with uninfected macrophages have been subtracted. The levels of IFN-γ obtained from cultures with uninfected macrophages ranged between 0.0 and 270.0 pg/ml and the levels of IL-5 from the same cultures were between 0.0 and 5.5 pg/ml. There are no significant differences (P > 0.05) between the different groups infected with dhfr-ts−, CC1 or LV39 concerning the production of IFN-γ or IL-5.
Importance of IL-12 in the generation of Th1 responses
IL-12 has received a great deal of attention in leishmaniasis, being responsible for the development of a Th1 response. Therefore, we determined whether IL-12 was produced in our IV system. We infected macrophages with either dhfr-ts−, CC1 or LV 39 and collected supernatants of the cultures 24, 48 or 96 h later. We could not detect IL-12 in any of these cultures (data not shown). However, when autologous PBL were cultured with Leishmania-infected macrophages for 7 days, IL-12 was produced (Fig. 4). No significant differences in IL-12 secretion were observed between the different groups infected with dhfr-ts−, CC1 or LV39 (P > 0.05).
Fig. 4.
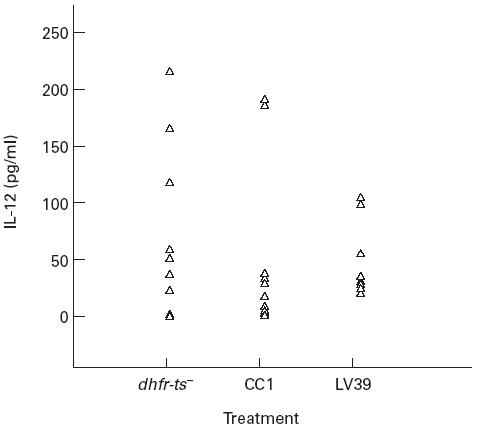
IL-12 in cultures with Leishmania-infected macrophages and non-adherent peripheral blood mononuclear cells (PBMC). Macrophages from 10 different donors were infected with either dhfr-ts−, CC1 or LV39 at a ratio of five parasites to one macrophage. After washing to remove free parasites, autologous non-adherent PBMC were added to the cultures and incubated for 7 days at 37°C, 5% CO2. At this point the supernatants were harvested and IL-12 levels were determined by an ELISA specific for the p40 and p70 subunits of IL-12. Concentrations of IL-12 produced in cultures with uninfected macrophages have been subtracted. The levels of IL-12 obtained from cultures with uninfected macrophages were between 9.0 and 70.0 pg/ml. There were no significant differences (P > 0.05) between the different groups infected with dhfr-ts−, CC1or LV39.
In order to determine the importance of IL-12 in the development of a Th1 response in our IV system, cultures were initiated in the presence or absence of recombinant human IL-12 or anti-human IL-12. The cytokine or antibody was added to cultures during the first stimulation of PBL, using infected macrophages as APC. After the primary stimulation, the blasts were harvested and restimulated with autologous infected macrophages as APC in the absence of either IL-12 or anti-IL-12. As shown in Fig. 5, IL-12 significantly increased the production of IFN-γ (P < 0.05) from cells of six donors tested (Fig. 5a). On the other hand, this cytokine led to a significant decrease (P < 0.05) in the secretion of IL-5 to levels that were non-detectable (Fig. 5b).
Fig. 5.
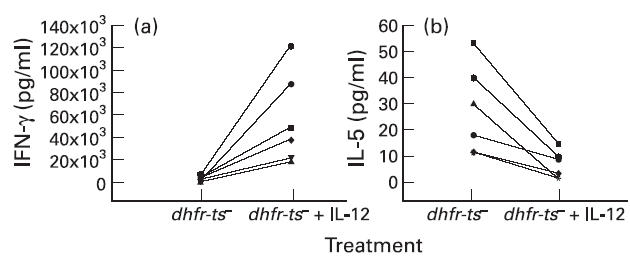
Effect of IL-12 on IFN-γ (a) and IL-5 (b) production obtained after stimulation of non-adherent peripheral blood mononuclear cells (PBMC) with autologous Leishmania-infected macrophages from normal human donors. Non-adherent PBMC from six different donors were exposed to autologous dhfr-ts−-infected macrophages for 7 days in the presence or absence of IL-12, and after this period the blast cells were restimulated with autologous dhfr-ts−-infected macrophages for 48 h. The supernatants were then collected and IFN-γ and IL-5 levels were determined by ELISA. The differences between the two groups of treatment (absence or presence of IL-12) are statistically significant (P < 0.05) concerning the secretion of IFN-γ and IL-5.
The role of endogenous IL-12 was also examined using anti-IL-12 during the first stimulation of PBL. As shown in Fig. 6, the presence of anti-IL-12 during the primary stimulation led to a significant decrease in the production of IFN-γ (Fig. 6a) from cells of all five donors tested (P < 0.05). In addition, anti-IL-12 enhanced production of IL-5 (Fig. 6b) from cells of the same donors (P < 0.05). It is interesting that the presence of this antibody during the primary stimulation of PBL from donors, who normally did not produce IL-5, induced the secretion of this cytokine, showing the importance of IL-12 in the generation of different Th responses.
Fig. 6.
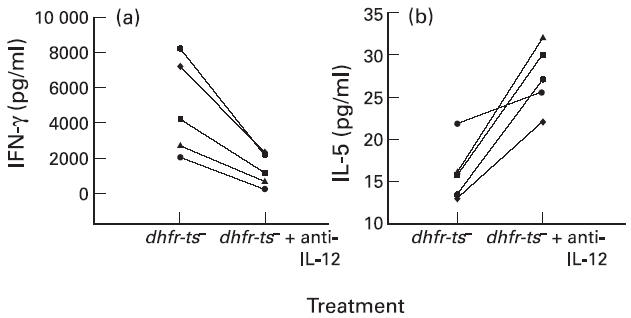
Effect of anti-IL-12 on IFN-γ (a) and IL-5 (b) production obtained after stimulation of non-adherent peripheral blood mononuclear cells (PBMC) with autologous Leishmania-infected macrophages from normal human donors. Non-adherent PBMC from five different donors were exposed to autologous dhfr-ts−-infected macrophages for 7 days in the presence or absence of anti-IL-12, and, after this period the blast cells were restimulated with autologous dhfr-ts−-infected macrophages for 48 h. The supernatants were then collected and IFN-γ and IL-5 levels were determined by ELISA. There are significant differences (P < 0.05) between the two groups of treatment (absence or presence of anti-IL-12) concerning the production of IFN-γ and IL-5.
DISCUSSION
The results of an in vitro priming system have been shown to correlate with in vivo responses in murine leishmaniasis [3]. Here we have succeeded in generating a system in which autologous infected macrophages serve as APC for priming PBL from normal human donors. This IV approach allowed us to examine interactions that occur between infected APC and PBL. Such interactions may influence the overall response to infection with Leishmania and therefore the outcome of disease. These studies are not possible using cells from patients with leishmaniasis since it is impossible to determine when the patient became infected with the parasite.
We analysed the ability of three different Leishmania parasites to prime T cells in the IV system: dhfr-ts−, CC1 and L. major (strain LV39). Although dhfr-ts− was engulfed by macrophages, it did not replicate inside the cells (Fig. 2). Interestingly, despite the fact dhfr-ts− did not replicate in macrophages, it elicited the same type of immune response (potentially protective IFN-γ with little IL-5) as did CC1 and LV39 (Fig. 3). Thus, since dhfr-ts− is avirulent but it induces a protective immune response, it may be an acceptable vaccine candidate for human use.
Evaluating cytokine production (IFN-γ and IL-5) in response to stimulation with parasite-infected macrophages, we observed that most donors elicited a Th1 or Th0 phenotype. Of 10 donors tested, six showed a Th1 profile, three Th0 and one was considered a non-secretor. Similar results were obtained by Russo et al. [8], who developed an in vitro system to study early responses from unexposed individuals to L. amazonensis infection. The authors obtained different cell lines from different donors and verified that most of them could be classified as Th1 or Th0 responders. Therefore, independent of the Leishmania used, human cells produce a similar cytokine profile.
Subauste et al. [9], studying the responses from unexposed individuals to Toxoplasma gondii infection in an in vitro model, observed an intense proliferation of CD4+αβ T cells, and these cells also secreted a significant amount of IFN-γ. Therefore, this rapid T cell response may play an important role in the early response to intracellular parasites. Indeed, recent work by Launois et al. [10] showed that very large amounts of cytokines (IL-4 in their work) are secreted by mouse αβ T cells within the first 16 h after infection with L. major. Finally, the results obtained in our experiments concerning the production of IFN-γ and IL-5 are similar to those observed in leishmaniasis patients; most of the patients with self-healing cutaneous leishmaniasis show strong Th1 responses [2,11] or, early in the infection, a Th0 pattern [12].
The Th1–Th2 dichotomy is probably influenced by the cytokines produced in the very early stages of Leishmania survival inside the macrophage. Upon entry into human macrophages, Leishmania induce contrasting signals leading to the production of tumour necrosis factor-alpha (TNF-α), which leads to macrophage activation, or to the production of transforming growth factor-beta (TGF-β) or IL-10, which are linked to macrophage deactivation and inhibition of IFN-γ [13,14]. The influence of IFN-γ produced by natural killer (NK) cells, which may depend on IL-12, is also involved in initial Th1 development [15]. Initial survival of Leishmania inside the macrophage probably depends on which of these or similar cytokines predominate in the microenvironment of infection. Our IV model allows study of these early events after phagocytosis of Leishmania by macrophages as well as modulation of immune responses through the addition of cytokines and anti-cytokines.
IL-12 has received a great deal of attention in leishmaniasis. The cytokine is produced following infection of mice with L. major [16,17] and it is important to control Th2 expansion and to promote the predominance of a Th1-type response [18–20]. In addition, treatment of susceptible mice with IL-12 renders them resistant [19,21,22]. Moreover, IL-12 has been used as an effective adjuvant for a killed vaccine for L. major [23]. In contrast to these in vivo results, L. major inhibits IL-12 production by infected murine macrophages in vitro [17,24]. Similar to these studies in mice, we could not detect secretion of IL-12 by L. major-infected macrophages, suggesting that the parasites do not induce the production of this cytokine by human macrophages. Interestingly, however, the addition of autologous PBL to infected macrophage cultures resulted in detectable production of IL-12 (Fig. 4). This suggests that signals delivered by the PBL, such as CD40–CD40L [25], might be important in the up-regulation of IL-12 production by human macrophages infected with L. major. It should be mentioned that in contrast to the results of this study with L. major, Russo et al. [8] observed IL-12 secretion by L. amazonensis-infected macrophages after 96 h of infection. This apparent difference could be explained by the use of different strains of Leishmania; the progression of infection by L. amazonensis in mice has been shown to be distinct from L. major [26].
Studies in human leishmaniasis confirm the relevant roles of IFN-γ and IL-12 as the major cytokines involved in host protection [1]. Therefore, studying the effects of IL-12 and anti-IL-12 in our IV system was important to understand the development of Leishmania-specific Th1 responses. As depicted in Fig. 5, IL-12 completely abrogated Th2 responses, increasing significantly the secretion of IFN-γ and abolishing the production of IL-5 by cells from the donors stimulated with Leishmania-infected macrophages. On the other hand, the presence of anti-IL-12 during the IV promoted a Th2 response, with a significant decrease in IFN-γ production and an increase in IL-5 (Fig. 6), showing the important role of IL-12 in the differentiation of human Th1 and Th2 responses. Therefore, the vaccine potential of dhfr-ts− for leishmaniasis may be further enhanced by transfecting the parasite with cytokine genes (e.g. IL-12) which favour the development of Th1 responses.
Acknowledgments
We thank Genetic Institute for providing human recombinant IL-12, Monica Estay for technical assistance and Dr Greg DeKrey and Dr Lamine Mbow for critical review of the manuscript. This study received financial support from NIH AI-29955 (R.G.T.), NIH AI 29646 and WHO Special Programme in Tropical Disease Research (S.M.B.). C.B. received a fellowship from CAPES/Fulbright in 1997 and from CAPES-Brasilia/Brazil in 1998.
REFERENCES
- 1.Barral-Netto M, Machado P, Bittencourt AL, Barral A. Recent advances in pathophysiology and treatment of human cutaneous leishmaniasis. Curr Opin Dermatol. 1997;4:51–8. [Google Scholar]
- 2.Carvalho EM, Correia Filho D, Bacellar O, Almeida RP, Rocha E. Characterization of the immune response in subjects with self-healing cutaneous leishmaniasis. Am J Trop Med Hyg. 1995;53:273–7. doi: 10.4269/ajtmh.1995.53.273. [DOI] [PubMed] [Google Scholar]
- 3.Titus RG, Gueiros-Filho FJ, Freitas LA, Beverley SM. Development of a safe live Leishmania vaccine line by gene replacement. Proc Natl Acad Sci USA 92. 1995;92:10267–71. doi: 10.1073/pnas.92.22.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankar AH, Titus RG. Leishmania-major-specific, CD4+, major histocompatibility complex class II-restricted T cells derived from lymphoid tissues of naive mice. J Exp Med. 1993;78:101–11. doi: 10.1084/jem.178.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veras P, Brodskyn CI, Balestieri FM, Freitas LAR, Ramos APS, Queiroz ARP, Beverly SM, Barral-Netto MA. dhfr-ts− Leishmania major knockout mutant cross protects against Leishmania amazonensis. Memorias do Instituto Osvaldo Cruz. 1999;94:491–6. doi: 10.1590/s0074-02761999000400011. [DOI] [PubMed] [Google Scholar]
- 6.Titus RG, Muller I, Kimsey P, Cerny A, Behin R, Zinkernagel RM, Louis JA. Exacerbation of experimental murine cutaneous leishmaniasis with CD4+Leishmania major-specific T cell lines or clones which secret IFN-γ and mediate parasite-specific delayed-type hypersensitivity. Eur J Immunol. 1991;21:559–67. doi: 10.1002/eji.1830210305. [DOI] [PubMed] [Google Scholar]
- 7.Goldroen MH, Gannon PJ, Lutz M, Holyoke ED. Isolation of human peripheral blood lymphocytes: modification of a double discontinuous density gradient of Ficoll–Hypaque. J Immunol Methods. 1977;14:15–7. doi: 10.1016/s0022-1759(97)90015-6. [DOI] [PubMed] [Google Scholar]
- 8.Russo DM, Chakrabarti P, Burns JM. Naive human T cells develop into Th1 or Th0 effectors and exhibit cytotoxicity early after stimulation with Leishmania-infected macrophages. J Infect Dis. 1998;77:1345–51. doi: 10.1086/515284. [DOI] [PubMed] [Google Scholar]
- 9.Subauste CS, Fuh F, de Waal M, Remington JS. Alpha beta T cell response to Toxoplasma gondii in previously individuals. J Immunol. 1998;160:3403–11. [PubMed] [Google Scholar]
- 10.Launois P, Maillard I, Pingel S, et al. IL-4 rapidly produced by Vβ4α8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 1997;6:541–9. doi: 10.1016/s1074-7613(00)80342-8. [DOI] [PubMed] [Google Scholar]
- 11.Pirmez C, Yamamura M, Uyemura K, Paes-Oliveira M, Conceicao-Silva F, Modlin RL. Cytokine patterns in the pathogenesis of human leishmaniasis. J Clin Invest. 1993;91:1390–5. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo DM, Burns JM, Carvalho EM, Armitage RJ, Grabstein KH, McMaster WR, Reed SG. Human T cells response to gp63, a surface antigen of Leishmania. J Immunol. 1991;147:3575–80. [PubMed] [Google Scholar]
- 13.Barral A, Teixeira M, Reis P, et al. Transforming growth factor beta in human cutaneous leishmaniasis. Am J Pathol. 1995;147:947–54. [PMC free article] [PubMed] [Google Scholar]
- 14.Barral-Netto M, Badaro R, Barral A, et al. Tumor necrosis factor (cachectin) in human visceral leishmaniasis. J Infec Dis. 1991;163:853–7. doi: 10.1093/infdis/163.4.853. [DOI] [PubMed] [Google Scholar]
- 15.Scharston-Kersten T, Scott P. The role of innate immune response in Th1 cell development following Leishmania major infection. J Leuk Biol. 1995;57:515–22. doi: 10.1002/jlb.57.4.515. [DOI] [PubMed] [Google Scholar]
- 16.Sypek JP, Chung CL, Mayor SE, et al. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiner SL, Zheng S, Wang ZE, Stowring L, Locksley RM. Leishmania promastigotes evade interleukin 12 (IL-12) and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med. 1994;179:447–56. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scharston-Kersten T, Afonso LC, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer activation and subsequent development in experimental leishmaniasis. J Immunol. 1995;154:5320–30. [PubMed] [Google Scholar]
- 19.Murray HW, Hariprashad J, Coffman RL. Behavior of visceral Leishmania donovani in an experimentally induced T helper 2 (Th2)-associated response model. J Exp Med. 1997;185:867–74. doi: 10.1084/jem.185.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinzel FP, Rerko RM, Ahmed F, Pearlman E. Endogenous IL-12 is required for control of Th2 cytokine responses capable of exacerbating leishmaniasis in normally resistant mice. J Immunol. 1995;155:730–9. [PubMed] [Google Scholar]
- 21.Heinzel FP, Rerko RM, Hatam F, Locksley RM. IL-2 is necessary for the progression of leishmaniasis in susceptible murine hosts. J Immunol. 1993;150:3924–31. [PubMed] [Google Scholar]
- 22.Wang ZE, Zheng S, Corry DB, Dalton DK, Seder RA, Reiner SL, Locksley RM. Interferon-γ-independent effects of interleukin 12 administered during acute or established infection due to Leishmania major. Proc Natl Acad Sci USA. 1994;91:12932–6. doi: 10.1073/pnas.91.26.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Afonso LC, Scahrton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin 12 in a vaccine against Leishmania major. Science. 1994;263:235–7. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 24.Carrera L, Gazzinelli RT, Badolato R, Hieny S, Muller W, Kuhn R, Sacks DL. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J Exp Med. 1996;183:515–26. doi: 10.1084/jem.183.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seguin R, Kasper LH. Sensitized lymphocytes and CD40 ligation augment interleukin 12 production by human dendritic cells in response to Toxoplasma gondii. J Infec Dis. 1999;179:467–74. doi: 10.1086/314601. [DOI] [PubMed] [Google Scholar]
- 26.Afonso LC, Scott P. Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect Immun. 1993;61:2952–7. doi: 10.1128/iai.61.7.2952-2959.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


