Abstract
This study shows that subcutaneous administration of increasing doses of IL-12, once a week, in 21 cancer patients increased the expression of cytokine genes (interferon-gamma (IFN-γ), tumour necrosis factor-alpha (TNF-α), IP-10, MIG, IL-10, IL-4) in peripheral blood mononuclear cells even at very low doses (30 ng/kg). Surprisingly, no circulating TNF-α or IL-4 could be detected in the plasma of patients treated with IL-12. However, a marked increase of soluble IL-4 receptor was demonstrated in the plasma of five of the six patients studied, which may represent an additional mechanism by which IL-12 inhibits the development of the Th2 response in vivo. A marked decline of IFN-γ and IP10 induction was recorded after repeated cycles of IL-12. In contrast, in most patients IL-12 increased IL-10 expression with no subsequent decrease during the course of therapy, and even an earlier peak of IL-10 induction at the 6th cycle. In addition, a constant up-regulation of serum soluble IFN-γ receptor levels was observed after each cycle of IL-12 treatment with a delayed peak compared with the IFN-γ peak. The constant rise of IL-10 and soluble IFN-γ receptor during IL-12 therapy may therefore contribute to the inhibition of IFN-γ activity detected after repeated cycles of IL-12. Lastly, a marked heterogeneity of cytokine induction was observed from one patient to another, which appeared to be independent of the dose of IL-12 administered. These data may lead to a better understanding of the biological activity of IL-12 and the in vivo mechanisms of its regulation.
Keywords: cytokine, IL-12, cancer, immunotherapy, soluble cytokine receptor
INTRODUCTION
IL-12 is a heterodimeric cytokine originally cloned from an Epstein–Barr virus-transformed cell line with an ability to activate natural killer (NK) cells and induce interferon-gamma (IFN-γ) production [1,2]. The main source of IL-12 is antigen-presenting cells (macrophages, dendritic cells), but other cells such as B lymphocytes, neutrophils, keratinocytes, and mast cells also produce this cytokine [3].
IL-12 has been shown to favour the development of the Th1 immune response and inhibit the differentiation of naive cells towards Th2 cells [4,5], as IL-12 gene deletion in mice results in markedly reduced Th1 responses [6]. Functional receptors for IL-12 among CD4 T cells appear to be restricted to recently activated uncommitted cells and Th1 cells, and are lost during differentiation of Th2 cells [7,8].
It has been reported in several animal tumour models that administration of IL-12 not only prevented the taking of grafted tumours, but also inhibited the development of established primary or metastatic tumours and induced anti-tumour immunity [9–11]. In humans, some clinical responses have been observed after either intratumour injection of fibroblasts engineered to release IL-12 or systemic administration of rhIL-12 [12,13].
Since IL-12 does not exert any direct effect on the control of tumour cell proliferation, other mechanisms have been proposed to explain its anti-tumour activity. Various groups have demonstrated that anti-IFN-γ MoAb abolishes the anti-tumour effect of IL-12 [14–16]. However, various studies suggest that other factors are required to explain the therapeutic effect of IL-12, as, in nude mice, IL-12 induced significant levels of IFN-γ without any anti-tumour activity [9]. Zilocchi et al. showed the ability of IFN-γ knock-out mice to reject tumour cells transduced with IL-12 [17]. Recently, Cui et al. reported that in mice only a subpopulation of NK1.1T cells, previously characterized as potent IL-4 producers, was responsible for the anti-tumour effect of IL-12 [18].
Various studies have also focused on IL-12-induced angiostatic factors, such as IP-10 and MIG, which may contribute to the anti-tumour activity of IL-12 [19].
One objective of the present study was therefore to assess the secondary release of cytokines, chemokines and soluble cytokine receptors in cancer patients enrolled in a phase I clinical trial of subcutaneous administration of rIL-12. Determination of these surrogate markers may help to define the in vivo biological activity of IL-12.
In addition, an unexpected toxicity was reported after the first phase II clinical trial of IL-12, requiring transient discontinuation of this therapy [20]. Administration of a predose of IL-12 markedly reduced this toxicity and was associated with a decrease of IL-12-induced IFN-γ [13,21–23]. In the course of the present study, we tried to analyse more clearly this attenuation phenomenon and characterize potential immunoregulators (IL-10, soluble IFN-γ receptor) possibly involved in the triggering and maintenance of this phenomenon.
PATIENTS AND METHODS
Patients
We analysed cytokine mRNA induction in peripheral whole blood derived from 21 cancer patients treated with increasing doses of rhIL-12 ranging from 30 ng/kg to 1500 ng/kg, administered by subcutaneous injection once a week until disease progression. These patients presented with 13 renal cell carcinomas, four melanomas, three sarcomas and one ovarian carcinoma.
Serum cytokine and soluble cytokine receptor levels were determined in nine patients enrolled in the two highest dose levels of IL-12 (1800 and 2100 ng/kg), corresponding to five renal cell carcinomas and four melanomas. This protocol was approved by a regional ethics committee and written informed consent was obtained from each subject according to institutional rules.
Clinical results of this protocol will be published elsewhere. All patients had metastatic disease and a Karnofsky status index ≥ 70.
Methods
Semiquantitative polymerase chain reaction
Total cellular RNA was extracted from peripheral whole blood using the RNA-zol B method based on the technique previously described by Chomczynski & Sacchi [24]. Total cellular RNA (2 μg) were mixed with oligo-dT primers to synthesize cDNA (First strand cDNA synthesis kit; Boehringer-Mannheim, Mannheim, Germany) according to the manufacturer's protocol.
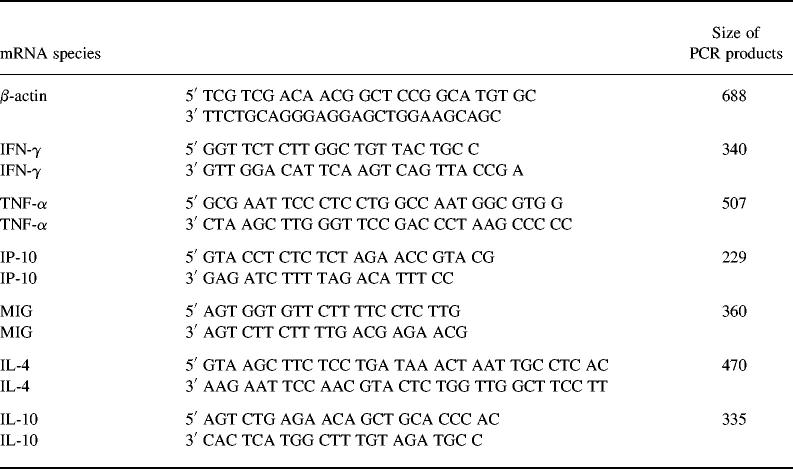
Polymerase chain reaction (PCR) was performed as previously described [25]. Table 1 gives the sequence of cytokine primers used in this study.
Table 1.
Sequences of 5′ and 3′ primers of nine cytokine target genes
For semiquantitative analysis, gels were scanned by densitometry and all results were normalized to β-actin gene expression.
Quantitative PCR
Quantitative titration of the various mRNAs was performed by reverse transcription PCR run to saturation, as previously described [25,26]. Briefly, one-tenth of the cDNA derived from mRNA was co-amplified to saturation with serial dilutions of appropriate internal standard which only differed by a 4-base pair deletion from the cDNA derived from the mRNA of interest. Amplification reactions were performed in a 50-μl mixture containing 50 U/ml of Taq polymerase (Promega, Charbonnieres, France), 200 μm dNTP, 0.2 μm of each of the two primers, 1.5 mm Mg2+, in Promega buffer. The reaction mixtures were overlaid with 50 μl of mineral oil. The PCR cycles were: 1 min at 94°C, 1 min annealing at 55°C and 1 min 30 s extension at 72°C. The reaction was performed in a Perkin-Elmer-Cetus DNA thermal cycler for 40 cycles and ended with a 10-min step at 72°C. A 2-μl aliquot of the amplified solution was then mixed in a final reaction volume of 10 μl containing 0.1 μm fluorescent primer, 20 U/ml Taq polymerase, 200 μm deoxynucleotide triphosphate and 3 mm Mg2+ in Promega buffer and submitted to one PCR cycle (run-off (RO) reaction). The sequences of IFN-γ and β-actin RO oligonucleotide primers were as follows: IFN-γ RO (TTGAAGTAAAAGGAGA CAATTTGGCTC), β-actin RO (AGGATGCCTCTCTTGCTC TG).
The RO reaction products were mixed with an equal volume of a 20-mm EDTA–95% formamide solution, heat-denatured at 80°C for 10 min and 2 μl of the resulting mixture were loaded on a 4% acrylamide–8 m urea gel and electrophoresed for 4 h on an automated DNA sequence analysis machine (373A DNA sequence; PE Applied Biosystem, Foster City, CA). Software was used to measure the length and area of each peak detected. The peak area ratio between known concentrations of standard DNA and target cDNA was used to determine the concentration of target cDNA derived from the mRNA to be quantified (Fig. 1).
Fig. 1.
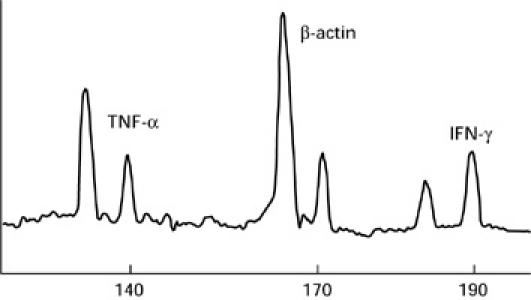
Fluorescence peak of wild-type cDNA and internal DNA standard for tumour necrosis factor-alpha (TNF-α), IFN-γ and β-actin. For each parameter, internal standard and target cDNA were co-amplified with the same specific primers. A run-off reaction with a third nested specific fluorescent primer was then performed and the polymerase chain reaction (PCR) product was loaded on an automated sequencer. The fluorescent profiles were recorded after electrophoresis. The peak area ratio between known concentrations of standard DNA and target DNA can be used to determine concentrations of wild-type cDNA.
Negative controls consisting of buffer alone (without cDNA) and a non-reverse transcribed sample RNA were included in each experiment.
To avoid PCR artefacts, aerosol-resistant pipette tips were used and distinguished pipettes dedicated to pre-PCR and post-PCR products.
In every case, the 5′ and 3′ primers had to span at least one intron in the genomic DNA sequence so that any amplification of contaminating genomic DNA would be easily identified.
Immunoassays
Levels of soluble IFN-γ receptor were determined using IFN-γ-R-specific ELISA developed by D. Novick (Rehovot, Israel) [27]. Briefly, 96 break-away, flat-bottomed wells (Nunc, Rochester, NY) microtitre plates were coated with 100 μl of anti-IFN-γ-R MoAb 177.10 (10 μg/ml) diluted in carbonate buffer (0.1 m Na2CO3/NaHCO3, pH 9.6) overnight at 4°C. The plates were then saturated with 200 μl of PBS containing 0.5% bovine serum albumin (BSA) and 0.05% Tween 20 (Merck, Schuchardt, Germany) for 1 h at room temperature. After washes with PBS–0.05% Tween 20, 100 μl of purified natural soluble IFN-γ receptor (provided by D. Novick) diluted in PBS–0.1% NP40 or serum were added and incubated for 3 h at 37°C. After washes, the plates were then incubated with 100 μl of anti-IFN-γ receptor polyclonal antibody—previously adsorbed by addition of 1% normal murine serum and human IgG (5 μg/ml)—overnight at 4°C. After washes, 100 μl of goat anti-rabbit polyclonal antibodies conjugated to horseradish peroxidase (Jackson Immunoresearch, West Grove, PA) diluted to 1:10 000 in PBS–0.05% Tween 20 were added for 2 h at room temperature. The reaction was revealed by addition of the peroxidase substrate (o-phenylenediamine dihydrochloride) and the optical density (OD) was read at a wavelength of 492 nm. The lowest concentration of human soluble IFN-γ receptor detected was 0.05 ng/ml. This test is not influenced by the presence of excess IFN-γ (D. Novick, data not shown).
Serum IFN-γ and tumour necrosis factor-alpha (TNF-α), levels were determined with a commercially available ELISA kit from Genzyme (Cambridge, MA.) and Endogen (Boston, MA), respectively.
IL-10, IP-10, IL-4 and soluble IL-4 receptor were measured using ELISA kits purchased from R&D (Minneapolis, MN). IL-4 at concentrations < 15 ng/ml did not interfere with soluble IL-4 receptor immunoassay and soluble IL-4 receptor did not interfere with IL-4 determination (R&D data).
RESULTS
Profile of cytokines induced after rhIL-12 administration
Induction of cytokines with potential anti-tumour activity
IL-12 did not exert its anti-tumour effect via direct interaction with tumour cells, but its activity may be mediated by cytotoxic factors such as IFN-γ and TNF-α. IL-12 induced an increase (more than two-fold) of IFN-γ and TNF-α mRNA expression in peripheral whole blood in 16 and 13 out of 21 patients, respectively (Table 2 and Fig. 1). Induction of these cytokines did not appear to be co-regulated. The increase in IFN-γ was often greater than five-fold and was sometimes more than 20-fold, while TNF-α induction was less intense (Table 2). The peak of IFN-γ induction was often observed early, before 24 h after IL-12 administration and a second peak was observed after a decline (Fig. 2). This second peak was observed in 13 of the 16 patients who exhibited a significant increase of IFN-γ mRNA after IL-12 administration. However, in most cases (12/13), the second peak was lower than the first peak (data not shown).
Table 2.
Induction of cytokine mRNA in peripheral whole blood after the first cycle of IL-12 administration
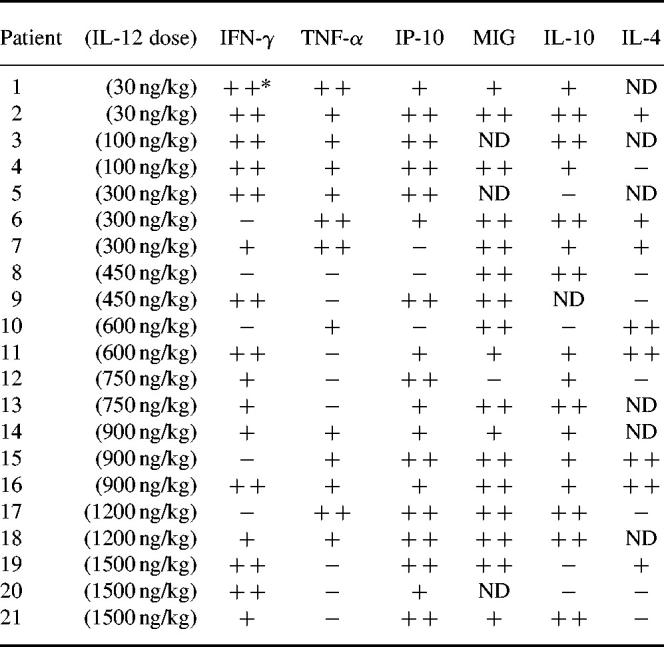
Polymerase chain reaction (PCR) was performed on samples collected before and 4, 12, 24, 48, 72 and 96 h after IL-12 administration.
*The relative increase of cytokine mRNA was determined from the ratio of specific cytokine mRNA/β-actin normalized densitometric values deduced from reverse transcriptase-PCR-amplified products obtained before and after IL-12 therapy. The maximum increase, regardless of the time after IL-12 administration, was selected.
+, Increase ranging between two-fold and five-fold; + +, greater than five-fold increase; −, less than two-fold increase; ND, not done.
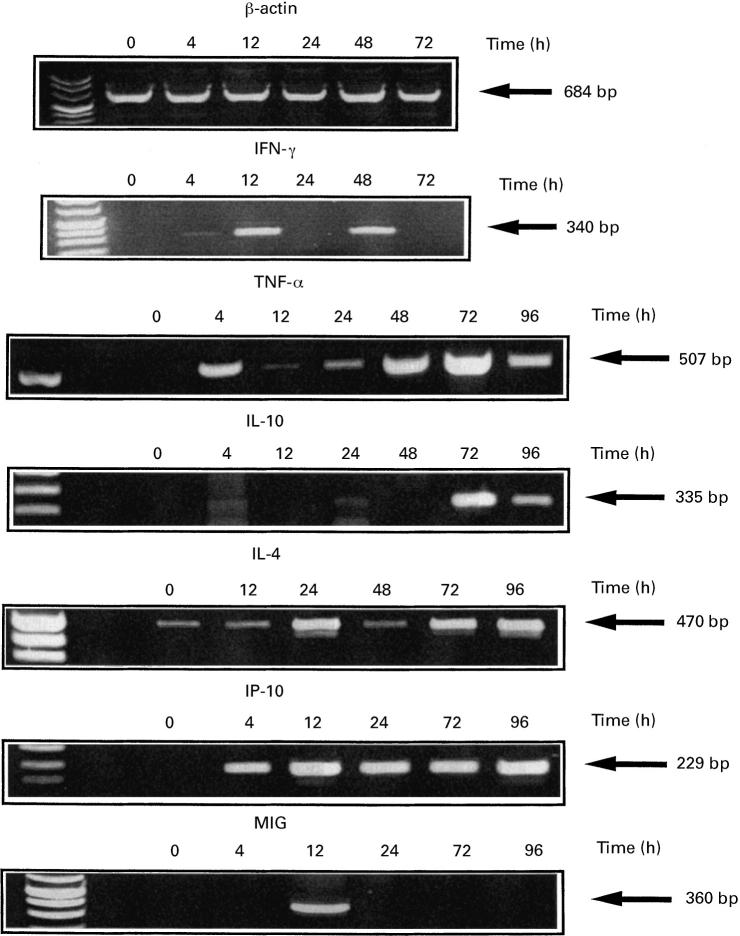
Fig. 2.
Profile of cytokine gene induction in peripheral whole blood of patients after rhIL-12 administration. rhIL-12 was administered by subcutaneous injection on day 0. β-actin, IFN-γ, tumour necrosis factor-alpha (TNF-α), IL-10, IL-4, IP-10 and MIG mRNA expression were assessed by semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR). Amplified products were loaded onto 2% agarose gel and stained with ethidium bromide. Gels were scanned by densitometry for semiquantitative analysis of mRNA induction. These results were derived from various illustrative patients treated with rhIL-12. See Table 2 for individual analysis.
Induction of cytokines with anti-angiogenesis activities
It has been claimed that the anti-tumour efficacy of IL-12 is dependent on its ability to inhibit angiogenesis [19]. In addition to the induction of IFN-γ, which is a potent angiostatic factor, we therefore focused on MIG and IP-10, which belong to a chemokine family with potent anti-angiogenesis features. We found that rIL-12 induced mRNA expression of IP-10 in 18 out of 21 patients and MIG mRNA in 17 out of 18 patients (Table 2). The long-lasting IP-10 mRNA expression contrasted with MIG mRNA induction, which was generally very transient, as illustrated in Fig. 2.
Induction of Th2 cytokines and soluble IL-4 receptor
Although IL-12 was considered to favour the development of Th1 immune response and to inhibit Th2 T cell differentiation, marked induction of IL-10 and IL-4 was observed in 80% (16/20) and 53% (8/15) of patients treated with rhIL-12, respectively (Table 2). In contrast to IFN-γ, the IL-10 mRNA peak was often delayed and never occurred before 40 h after the start of IL-12 injection (Fig. 2), which supports its role as a negative regulator of IL-12 activity.
IL-10 induction was confirmed at the protein level (see below), while no IL-4 could be detected by ELISA in the plasma of six cancer patients treated with high-dose IL-12 (Fig. 3). Surprisingly, we found a marked increase of soluble IL-4 receptor in the plasma of five of the six patients in whom no IL-4 was detected (Fig. 3). Peak induction generally occurred 72 h after IL-12 administration (Fig. 3). Soluble IL-4 receptor did not interfere with IL-4 determination by the ELISA kit used (data not shown).
Fig. 3.
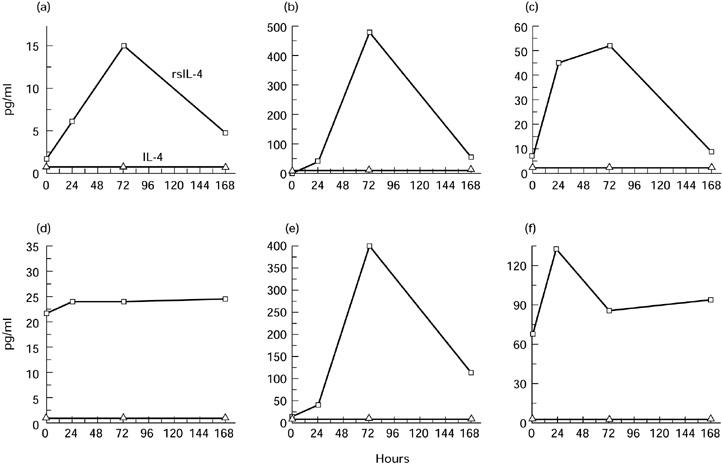
Plasma IL-4 and soluble IL-4 receptor levels in patients treated with rhIL-12. Plasma was collected at various times after subcutaneous rhIL-12 administration. (a,b,c) Patients receiving 1800 ng/kg of IL-12. (d,e,f) Patients treated with 2100 ng/kg of IL-12. IL-4 and soluble IL-4 receptor were measured by ELISA.
We did not find any significant change of IL-11 and transforming growth factor-beta 1 (TGF-β1) mRNA, known to act as negative regulators of IL-12 activity [28,29], in peripheral whole blood of IL-12-treated patients (data not shown).
Minimal dose of rhIL-12 required to induce in vivo changes in cytokine gene expression
The lowest dose of subcutaneous administered IL-12 (30 ng/kg) was sufficient to induce gene expression of cytokines such as IFN-γ, TNF-α, IP-10 or IL-10 from peripheral whole blood of the two patients tested (Table 2). Induction of cytokine gene expression therefore appeared to be a sensitive marker to assess in vivo biological activities of rhIL-12, as, for example, no modification of peripheral blood mononuclear cells (PBMC) was observed in the same patient (data not shown).
Lack of relationship between IL-12 dose levels and IFN-γ mRNA and protein induction
Semiquantitative PCR failed to demonstrate any clear correlation between the increase of cytokine gene expression in peripheral whole blood and the dose of IL-12 administered (100 ng/kg to 1500 ng/kg) (Table 1). To confirm this observation, we measured cytokine mRNA by a quantitative PCR assay in 10 patients in whom mRNA was still available and in whom an increase of IFN-γ mRNA was detected by semiquantitative PCR. The time of analysis chosen for each patient corresponded to peak induction of IFN-γ mRNA, as observed on semiquantitative PCR (Fig. 4a). The number of β-actin transcripts was also quantified and subsequently used to normalize IFN-γ. As shown in Fig. 4a, no linear relationship was demonstrated between IFN-γ mRNA copies in peripheral whole blood after IL-12 administration and the dose of cytokine administered. IFN-γ mRNA values after IL-12 administration ranged between 0.5 and 90 copies per 106 mRNA β-actin copies.
Fig. 4.
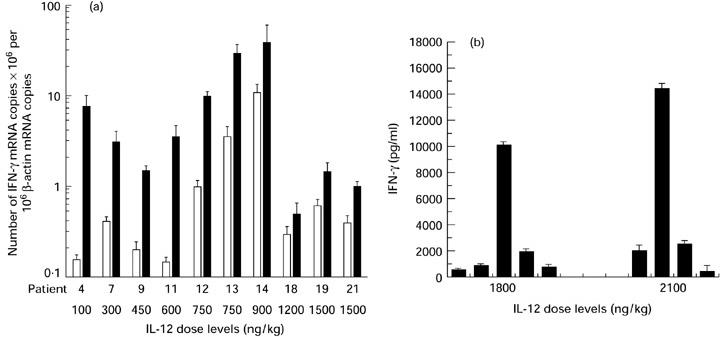
Analysis of the relationship between IL-12 dose levels and IFN-γ mRNA and protein induction. (a) Number of IFN-γ mRNA copies assessed by quantitative polymerase chain reaction (PCR) in peripheral whole blood derived from cancer patients before (□) and after (▪) treatment with rhIL-12. The time of analysis chosen for each patient corresponded to the time of peak IFN-γ mRNA induction after rhIL-12 administration, as observed on semiquantitative PCR. All results were normalized to β-actin gene expression. Data are expressed as mean ± s.d. (bars). (b) Plasma IFN-γ levels were determined by ELISA in the two patient groups treated with the highest rhIL-12 dose levels. The plasma IFN-γ peak is shown for each patient. Data are the means of triplicate determinations ± s.d. (bars).
For the last two patient groups (1800 ng/kg and 2100 ng/kg dose levels), plasma IFN-γ was measured by ELISA. As previously reported for IFN-γ mRNA induction, the intensity of the IFN-γ peaks was very variable, ranging from 420 pg/ml to > 10 000 pg/ml (Fig. 4b). No direct relationship between the dose of IL-12 and the peak IFN-γ value was demonstrated, as low IFN-γ induction (< 500 pg/ml) and high IFN-γ induction (> 10 000 pg/ml) were observed for the two dose levels (Fig. 4b).
Analysis of the attenuation phenomenon after repeated cycles of IL-12 administration
The peak plasma IFN-γ concentration decreased during the 6th cycle of high-dose IL-12 administration (1800 and 2100 ng/kg) in all six patients analysed, compared with the first cycle (Fig. 5). To assess the biological relevance of these findings, we studied plasma levels of IP-10, an IFN-γ-inducible protein. A decrease of IP-10 induction was also recorded after repeated cycles of IL-12 in the same group of patients (Fig. 5). These results therefore suggest that the decrease in IFN-γ concentration was also associated with a reduction of its biological activity.
Fig. 5.
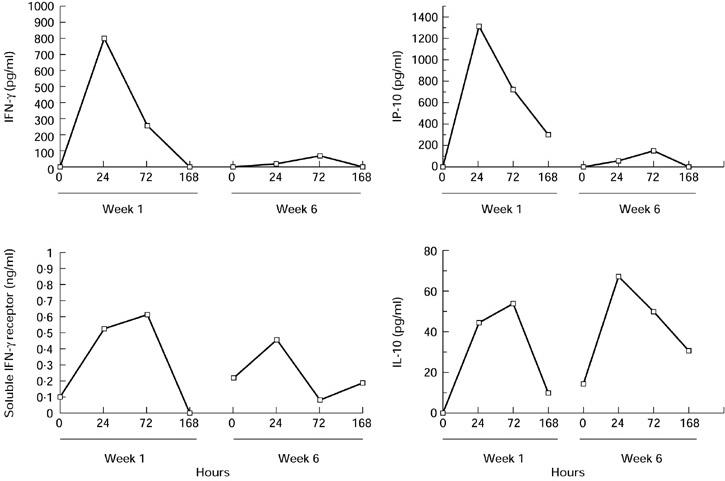
Analysis of the attenuation phenomenon after repeated cycles of IL-12 administration. Plasma was collected from six cancer patients at various times during the first and sixth weeks of IL-12 administration. IFN-γ, IP-10, IL-10 and soluble IFN-γ receptor concentrations were measured by ELISA. The results shown are derived from one patient and are representative of the profile observed in the patient group.
To investigate the mechanisms possibly contributing to this phenomenon, we analysed the plasma concentrations of two potential inhibitors of IFN-γ production and activity: IL-10 and soluble IFN-γ receptor.
In the same group of six patients, IL-12 always increased the plasma IL-10 concentration with no decrease during therapy (Fig. 5). As expected during the first cycle, the peak serum IL-10 level generally occurred 72 h after IL-12 administration, in line with kinetic results obtained for mRNA levels. In contrast, the maximal increase appeared earlier (24 h) in 5/6 patients during the 6th cycle of IL-12 administration. We also observed up-regulation of basal plasma IL-10 levels before the 6th cycle of IL-12 administration compared with pretreatment IL-10 values (Fig. 5).
A constant rise of serum soluble IFN-γ receptor levels was also observed in the same group of six patients with a delayed peak compared with the IFN-γ peak (Fig. 5). The intensity of induction of soluble IFN-γ receptor after repeated cycles of IL-12 was not attenuated in four out of six patients. Basal serum soluble IFN-γ receptor levels just before the 6th cycle of IL-12 administration were increased in four out of six patients tested compared with pretreatment values (Fig. 5).
To assess whether this state of desensitization to IL-12-induced IFN-γ also occurred at the mRNA level, we analysed IFN-γ mRNA induction in peripheral whole blood derived from 12 cancer patients during the 6th cycle of IL-12 administration. A marked induction (more than two-fold increase) of IFN-γ mRNA was observed in only 5/12 (40%) patients compared with 76% of patients during the first cycle (data not shown).
DISCUSSION
In this study, we show that rIL-12 administration in humans induces an increase of various cytokine mRNAs (IFN-γ, IP-10, TNF-α...) which could constitute surrogate markers to assess the in vivo biological activity of IL-12, as a dose of IL-12 as low as 30 ng/kg was sufficient to induce cytokine mRNAs in peripheral whole blood (Table 2). This increased IFN-γ mRNA was expected, as IL-12 is considered to be one of the main regulators of IFN-γ mRNA expression [6]. We found an early peak of mRNA only 4 h after IL-12 administration (Fig. 1) in line with Tannenbaum's results in mice, showing that intratumour induction of IFN-γ could be detected only 4 h after IL-12 administration [30]. Since T lymphocytes and NK cells are considered to be the main sources of IFN-γ, the lymphocytopenia occurring at the beginning of IL-12 therapy could explain the failure to detect mRNA in peripheral whole blood in a few cases (Table 2). This transient lymphocytopenia may therefore introduce bias in the analysis of surrogate markers produced by T lymphocytes.
In contrast, we observed induction of TNF-α and IL-4 mRNA in the peripheral whole blood of 13/21 (62%) and 8/15 (53%) patients treated with IL-12 (Table 2), but no TNF-α or IL-4 protein could be detected in the serum of patients treated with high-dose IL-12 (Fig. 3 and data not shown). IL-12 administration in mice stimulates the production of TNF-α mRNA and protein, but inhibits IL-4 gene expression [22,31]. However, even when TNF-α induction was demonstrated in mice, it was weak and often failed to reach twice the background levels [22]. In previous clinical trials, most teams failed to detect TNF-α in serum [13,32]. Interference of soluble TNF-α and IL-4 receptors on serum TNF-α and IL-4 assays could account for the discrepancies between cytokine mRNA and protein expression. However, neither soluble TNF-α nor IL-4 receptor were shown to interfere with the test used to detect TNF-α or IL-4 (R&D data). In addition, during clinical follow up of patients, the absence of toxicity possibly related to circulating serum TNF-α did not suggest the presence of high serum TNF-α concentrations in patients treated with IL-12. Our results obviously do not exclude the possible synthesis of TNF-α as a precursor protein not cleaved by metalloproteases and released into serum [33,34].
It is noteworthy that these opposite results for mRNA and protein determination have been previously reported for IL-4 and other cytokines [35,36].
We found a significant induction of chemokines (IP-10, MIG) regulated by IFN-γ with known angiostatic effects. Up-regulation of IP-10 was confirmed at both the mRNA and protein levels (Table 2, Fig. 5), supporting the role of IL-12 in inhibiting angiogenesis, observed in murine models. A direct effect of IP-10 and MIG to control the growth of human tumours transplanted into nude mice was also recently demonstrated [37–39].
Although the MIG and IP-10 genes are highly homologous IFN-inducible proteins, the difference observed in the kinetics of IP-10 and MIG mRNA induction after IL-12 administration could be explained by the difference in their pattern of expression and their response to inducers, as MIG is selectively and exclusively induced by IFN-γ, whereas IP-10 could be up-regulated by agents other than IFN-γ (TNF-α, IL-1α) [40–42] which may contribute to its sustained expression after IL-12 administration, as observed by other authors [38].
An original observation drawn from these results concerns the marked increase of soluble IL-4 receptor in patients treated by IL-12. Although many cell types are able to constitutionally produce low levels of sIL-4R, sIL-4R production has been shown to be significantly up-regulated in vitro by T cell activation and IL-4 [43]. Although anti-IL-4 MoAb antibodies reduced sIL-4R induction after T cell activation, suggesting a role of endogenous IL-4 in the regulation of sIL-4R, Blum et al. clearly demonstrated the ability of IL-4 knock-out mice to release sIL-4R following anti-CD3 or phorbol myristate acetate (PMA) activation [44]. Even in the absence of IL-4 detected in the serum of IL-12-treated patients, other putative regulators of sIL-4R could therefore mediate this induction. The role of IFN-γ in the regulation of sIL-4R is open to debate, as in one model IFN-γ had no significant effect on either the basal or rIL-4-induced sIL-4R production in whole spleen, while in mature bone marrow-derived macrophages sIL-4R expression was induced by exposure to IFN-γ [43,45]. Since sIL-4R behaves like a natural antagonist of IL-4 when present in a large excess over IL-4 [46], as observed after IL-12 treatment, induction of sIL-4R may represent an additional mechanism by which IL-12 inhibits development of the Th2 response in vivo.
The plasma IFN-γ peak decreased during the 6th cycle of IL-12 therapy. This attenuated IFN-γ response after previous exposure to IL-12 has been previously reported by various groups [13,23,32]. Interestingly, in clinical trials including a predose of IL-12, corresponding to sensitization of the patient to this cytokine, the decline in IFN-γ concentration seemed to be associated with the absence of toxicity of IL-12 [21]. It is unlikely that the attenuated response is solely due to changes in IL-12 receptor expression, as IFN-γ production in response to the T cell mitogen concanavalin A (Con A) was also decreased by IL-12 pretreatment [21]. In addition, other IL-12-inducible molecules, such as corticosterone or neopterin, were not attenuated after multiple cycles of IL-12 [21,22]. In order to provide some clues to explain this phenomenon, we first showed that the decrease of IFN-γ also occurred at the mRNA level and that IFN-γ-regulated molecules such as IP-10 were also inhibited after repeated cycles of IL-12. These data suggest that an early event in a specific pathway leading to IFN-γ induction is blocked after chronic IL-12 administration. In contrast, the plasma concentration of IL-10, a known inhibitor of IFN-γ production and IL-12 activity [47,48], did not decrease during IL-12 therapy (Fig. 5). In humans, Bajetta et al. also revealed increased IL-10 production after IL-12 administration, with peak serum IL-10 levels observed during the second cycle of treatment [32]. However, up-regulation of basal plasma IL-10 levels before the 6th cycle of IL-12 administration compared with pretreatment IL-10 values, and the earlier peak of plasma IL-10 levels after the 6th cycle of administration, provide new arguments in favour of the role of IL-10 in this attenuation phenomenon. It must be emphasized that anti-IL-10 antibodies reduced the protective effect of a predose of IL-12 in mice [22].
In our study, an increase of serum soluble IFN-γ receptor levels was observed after IL-12 treatment, with a delayed peak compared with the IFN-γ peak and no clear attenuation after repeated cycles. This potential inhibitor of IFN-γ could therefore contribute to the down-regulation of IFN-γ activity. The increase of sIFN-γ receptor despite down-regulation of serum IFN-γ levels after chronic IL-12 administration suggests that, like other soluble cytokine receptors, sIFN-γ release may be regulated by agents other than its own ligand [45,49].
Lastly, a striking result of this study concerns the marked heterogeneity of cytokine induction from one patient to another, which appeared to be independent of the dose of IL-12. These results were confirmed both in terms of mRNA and protein, as IFN-γ mRNA copies after IL-12 treatment ranged between 0.5 and 90 copies per 106 β-actin mRNA copies (Fig. 4). The intensity of the plasma IFN-γ peak was very variable, ranging from 420 pg/ml to > 10 000 pg/ml for the same dose level (Fig. 4b). This absence of correlation between the dose of IL-12 administered and the in vivo biological activity of IL-12 indicates that, in contrast with chemotherapy or conventional drugs, the highest dose did not always correspond to the highest activity.
Various factors could contribute to this heterogeneity. First, it has been reported that the genetic background, such as cytokine gene polymorphism, is correlated with the in vitro level of cytokine production [50–52]. Second, the patient's immunological status may interfere with the host response to immunoregulators. For instance, in vitro, IL-12 prevents the generation of IL-4-producing cells from naive cells derived from wild-type mice, but in various murine models IL-12 accentuates an established Th2 response [53–56].
Although the secondary induction of cytokines participates in the antitumour activity of IL-12 or other immunomodulators, the heterogeneity of the biological response after their administration without a marked in vivo dose effect, may explain the absence of a clear relationship between clinical response and the dose of cytokine administered in previous clinical trials [57–59]. Determination of cytokine levels may sometimes be helpful to select patients most likely to respond to these treatments [60–62].
Acknowledgments
This work was supported by Institut Curie, INSERM, Université Pierre and Marie Curie and grants from Ligue Nationale contre le Cancer and Indo-French Centre for the Promotion of advanced research (IFCPAR).
REFERENCES
- 1.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–45. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern AS, Podlaski FJ, Hulmes JD, et al. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci USA. 1990;87:6808–12. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 4.Manetti R, Parronchi P, Giudizi MG, et al. Natural killer cell stimulatory factor (interleukin 12 +IL-12-) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'garra A, Murphy KM. Development of Th1, CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 6.Magram J, Connaughton SE, Warrier RR, et al. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–81. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 7.Rogge L, Barberis-Maino L, Biffi M, et al. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–31. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gately MK, Renzetti LM, Magram J, et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 9.Brunda MJ, Luistro L, Warrier RR, et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178:1223–30. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vagliani M, Rodolfo M, Cavallo F, et al. Interleukin 12 potentiates the curative effect of a vaccine based on interleukin 2-transduced tumor cells. Cancer Res. 1996;56:467–70. [PubMed] [Google Scholar]
- 11.Chen L, Chen D, Block E, O'donnell M, Kufe DW, Clinton SK. Eradication of murine bladder carcinoma by intratumor injection of a bicistronic adenoviral vector carrying cDNAs for the IL-12 heterodimer and its inhibition by the IL-12 p40 subunit homodimer. J Immunol. 1997;159:351–9. [PubMed] [Google Scholar]
- 12.Lotze MT, Zitvogel L, Campbell R, et al. Cytokine gene therapy of cancer using interleukin-12: murine and clinical trials. Ann NY Acad Sci. 1996;795:440–54. doi: 10.1111/j.1749-6632.1996.tb52715.x. [DOI] [PubMed] [Google Scholar]
- 13.Atkins MB, Robertson MJ, Gordon MH, et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res. 1997;3:409–17. [PubMed] [Google Scholar]
- 14.Nastala CL, Edington HD, McKinney TG, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994;153:1697–706. [PubMed] [Google Scholar]
- 15.Zou JP, Yamamoto N, Fujii T, et al. Systemic administration of rIL-12 induces complete tumor regression and protective immunity: response is correlated with a striking reversal of suppressed IFN-gamma production by anti-tumor T cells. Int Immunol. 1995;7:1135–45. doi: 10.1093/intimm/7.7.1135. [DOI] [PubMed] [Google Scholar]
- 16.Brunda MJ, Luistro L, Hendrzak JA, Fountoulakis M, Garotta G, Gately MK. Role of interferon-gamma in mediating the antitumor efficacy of interleukin-12. J Immunother Emphasis Tumor Immunol. 1995;17:71–7. doi: 10.1097/00002371-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Zilocchi C, Stoppacciaro A, Chiodoni C, Parenza M, Terrazzini N, Colombo MP. Interferon gamma-independent rejection of interleukin 12-transduced carcinoma cells requires CD4+ T cells and granulocyte/macrophage colony-stimulating factor. J Exp Med. 1998;188:133–43. doi: 10.1084/jem.188.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui J, Shin T, Kawano T, et al. Requirement for Valpha14 NK T cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 19.Voest EE, Kenyon BM, O'reilly MS, Truitt G, d'amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87:581–6. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. IL-12 deaths: explanation and a puzzle. Science. 1995;270:908. doi: 10.1126/science.270.5238.908a. [DOI] [PubMed] [Google Scholar]
- 21.Leonard JP, Sherman ML, Fisher GL, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90:2541–8. [PubMed] [Google Scholar]
- 22.Sacco S, Heremans H, Echtenacher B, et al. Protective effect of a single interleukin-12 (IL-12) predose against the toxicity of subsequent chronic IL-12 in mice: role of cytokines and glucocorticoids. Blood. 1997;90:4473–9. [PubMed] [Google Scholar]
- 23.Coughlin CM, Wysocka M, Trinchieri G, Lee WM. The effect of interleukin 12 desensitization on the antitumor efficacy of recombinant interleukin 12. Cancer Res. 1997;57:2460–7. [PubMed] [Google Scholar]
- 24.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 25.Tartour E, Gey A, Sastre-Garau X, et al. Analysis of interleukin 6 gene expression in cervical neoplasia using a quantitative polymerase chain reaction assay: evidence for enhanced interleukin 6 gene expression in invasive carcinoma. Cancer Res. 1994;54:6243–8. [PubMed] [Google Scholar]
- 26.Tartour E, Gey A, Sastre-Garau X, Lombard Surin I, Mosseri V, Fridman WH. Prognostic value of intratumoral interferon gamma messenger RNA expression in invasive cervical carcinomas. J Natl Cancer Inst. 1998;90:287–94. doi: 10.1093/jnci/90.4.287. [DOI] [PubMed] [Google Scholar]
- 27.Toren A, Barak V, Novick D, Nagler A. Soluble interferon-gamma receptor and interferon-gamma in patients undergoing allogeneic bone marrow transplantation for hematological malignancies. Cytokines Cell Mol Ther. 1997;3:153–8. [PubMed] [Google Scholar]
- 28.Leng SX, Elias JA. Interleukin-11 inhibits macrophage interleukin-12 production. J Immunol. 1997;159:2161–8. [PubMed] [Google Scholar]
- 29.Bellone G, Aste-Amezaga M, Trinchieri G, Rodeck U. Regulation of NK cell functions by TGF-beta 1. J Immunol. 1995;155:1066–7. [PubMed] [Google Scholar]
- 30.Tannenbaum CS, Wicker N, Armstrong D, et al. Cytokine and chemokine expression in tumors of mice receiving systemic therapy with IL-12. J Immunol. 1996;156:693–9. [PubMed] [Google Scholar]
- 31.Morris SC, Madden KB, Adamovicz JJ, et al. Effects of IL-12 on in vivo cytokine gene expression and Ig isotype selection. J Immunol. 1994;152:1047–56. [PubMed] [Google Scholar]
- 32.Bajetta E, Del Vecchio M, Mortarini R, et al. Pilot study of subcutaneous recombinant human interleukin 12 in metastatic melanoma. Clin Cancer Res. 1998;4:75–85. [PubMed] [Google Scholar]
- 33.Moss ML, Jin SL, Milla ME, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–6. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 34.Solomon KA, Covington MB, deCicco CP, Newton RC. The fate of pro-TNF-alpha following inhibition of metalloprotease-dependent processing to soluble TNF-alpha in human monocytes. J Immunol. 1997;159:4524–31. [PubMed] [Google Scholar]
- 35.Rivas D, Mozo L, Zamorano J, et al. Upregulated expression of IL-4 receptors and increased levels of IL-4 in rheumatoid arthritis patients. J Autoimmun. 1995;8:587–600. doi: 10.1016/0896-8411(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 36.Michon JM, Gey A, Moutel S, Tartour E, Meresse V, Fridman W, Teillaud JL. In vivo induction of functional Fc gammaRI (CD64) on neutrophils and modulation of blood cytokine mRNA levels in cancer patients treated with G-CSF (rMetHuG-CSF) Br J Haematol. 1998;100:550–6. doi: 10.1046/j.1365-2141.1998.00597.x. [DOI] [PubMed] [Google Scholar]
- 37.Sgadari C, Farber JM, Angiolillo AL, et al. Mig, the monokine induced by interferon-gamma, promotes tumor necrosis in vivo. Blood. 1997;89:2635–43. [PubMed] [Google Scholar]
- 38.Sgadari C, Angiolillo AL, Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood. 1996;87:3877–82. [PubMed] [Google Scholar]
- 39.Arenberg DA, Kunkel SL, Polverini PJ, et al. Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med. 1996;184:981–92. doi: 10.1084/jem.184.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amichay D, Gazzinelli RT, Karupiah G, Moench TR, Sher A, Farber JM. Genes for chemokines MuMig and Crg-2 are induced in protozoan and viral infections in response to IFN-gamma with patterns of tissue expression that suggest nonredundant roles in vivo. J Immunol. 1996;157:4511–20. [PubMed] [Google Scholar]
- 41.Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–6. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 42.Ohmori Y, Hamilton TA. Cell type and stimulus specific regulation of chemokine gene expression. Biochem Biophys Res Commun. 1994;198:590–6. doi: 10.1006/bbrc.1994.1086. [DOI] [PubMed] [Google Scholar]
- 43.Chilton PM, Fernandez-Botran R. Production of soluble IL-4 receptors by murine spleen cells is regulated by T cell activation and IL-4. J Immunol. 1993;151:5907–17. [PubMed] [Google Scholar]
- 44.Blum H, Wolf M, Enssle K, Rollinghoff M, Gessner A. Two distinct stimulus-dependent pathways lead to production of soluble murine interleukin-4 receptor. J Immunol. 1996;157:1846–53. [PubMed] [Google Scholar]
- 45.Ruhl S, Pluznik DH, Feldman GM. Soluble interleukin-4 receptor production by murine myeloid progenitor cells: induction by interleukin-6 and interferon-gamma. Cytokine. 1993;5:144–9. doi: 10.1016/1043-4666(93)90053-8. [DOI] [PubMed] [Google Scholar]
- 46.Fanslow WC, Clifford KN, Park LS, et al. Regulation of alloreactivity in vivo by IL-4 and the soluble IL-4 receptor. J Immunol. 1991;147:535–40. [PubMed] [Google Scholar]
- 47.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–9. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.d'andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–8. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hart PH, Hunt EK, Bonder CS, Watson CJ, Finlay-Jones JJ. Regulation of surface and soluble TNF receptor expression on human monocytes and synovial fluid macrophages by IL-4 and IL-10. J Immunol. 1996;157:3672–80. [PubMed] [Google Scholar]
- 50.Pociot F, Briant L, Jongeneel CV, et al. Association of tumor necrosis factor (TNF) and class II major histocompatibility complex alleles with the secretion of TNF-alpha and TNF-beta by human mononuclear cells: a possible link to insulin-dependent diabetes mellitus. Eur J Immunol. 1993;23:224–31. doi: 10.1002/eji.1830230135. [DOI] [PubMed] [Google Scholar]
- 51.Turner D, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24:1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 52.Danis VA, Millington M, Hyland VJ, Grennan D. Cytokine production by normal human monocytes: inter-subject variation and relationship to an IL-1 receptor antagonist (IL-1Ra) gene polymorphism. Clin Exp Immunol. 1995;99:303–10. doi: 10.1111/j.1365-2249.1995.tb05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wynn TA, Eltoum I, Oswald IP, Cheever AW, Sher A. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med. 1994;179:1551–61. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Afonso LC, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–7. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 55.Wang ZE, Zheng S, Corry DB, et al. Interferon gamma-independent effects of interleukin 12 administered during acute or established infection due to Leishmania major. Proc Natl Acad Sci USA. 1994;91:12932–6. doi: 10.1073/pnas.91.26.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Finkelman FD, Madden KB, Cheever AW, et al. Effects of interleukin 12 on immune responses and host protection in mice infected with intestinal nematode parasites. J Exp Med. 1994;179:1563–72. doi: 10.1084/jem.179.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tartour E, Fridman WH. Cytokines and cancer. Int Rev Immunol. 1998;16:683–704. doi: 10.3109/08830189809043014. [DOI] [PubMed] [Google Scholar]
- 58.Tahara H, Zeh HJ, III, Storkus WJ, et al. Fibroblasts genetically engineered to secrete interleukin 12 can suppress tumor growth and induce antitumor immunity to a murine melanoma in vivo. Cancer Res. 1994;54:182–9. [PubMed] [Google Scholar]
- 59.Schmidt W, Schweighoffer T, Herbst E, et al. Cancer vaccines: the interleukin 2 dosage effect. Proc Natl Acad Sci USA. 1995;92:4711–4. doi: 10.1073/pnas.92.10.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartzentruber DJ. In vitro predictors of clinical response in patients receiving interleukin-2 based immunotherapy. Curr Opin Oncol. 1993;5:1055–8. doi: 10.1097/00001622-199311000-00018. [DOI] [PubMed] [Google Scholar]
- 61.Herrmann F, Helfrich SG, Lindemann A, Schleiermacher E, Huber C, Mertelsmann R. Elevated circulating levels of tumor necrosis factor predict unresponsiveness to treatment with interferon alfa-2b in chronic myelogenous leukemia. J Clin Oncol. 1992;10:631–4. doi: 10.1200/JCO.1992.10.4.631. [DOI] [PubMed] [Google Scholar]
- 62.Tartour E, Blay JY, Dorval T, et al. Predictors of clinical response to interleukin 2 based immunotherapy in melanoma patients: a French multiinstitutional study. J Clin Oncol. 1996;14:1697–703. doi: 10.1200/JCO.1996.14.5.1697. [DOI] [PubMed] [Google Scholar]