Abstract
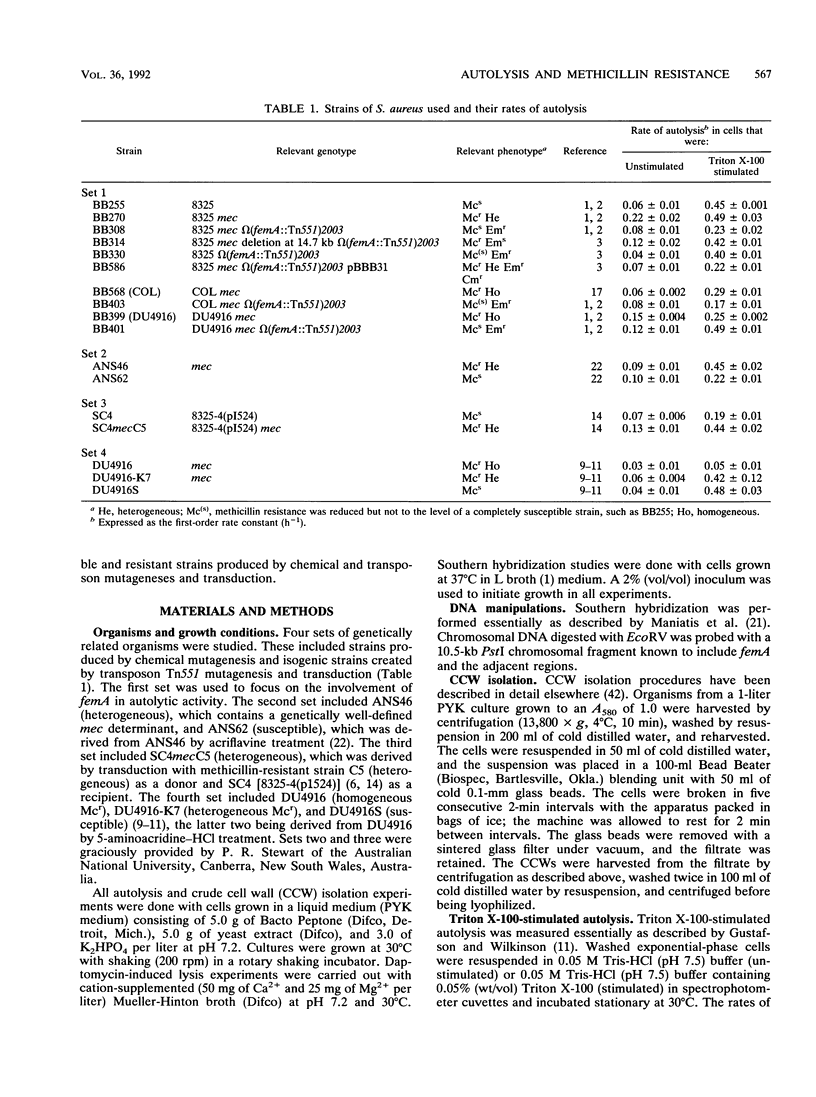
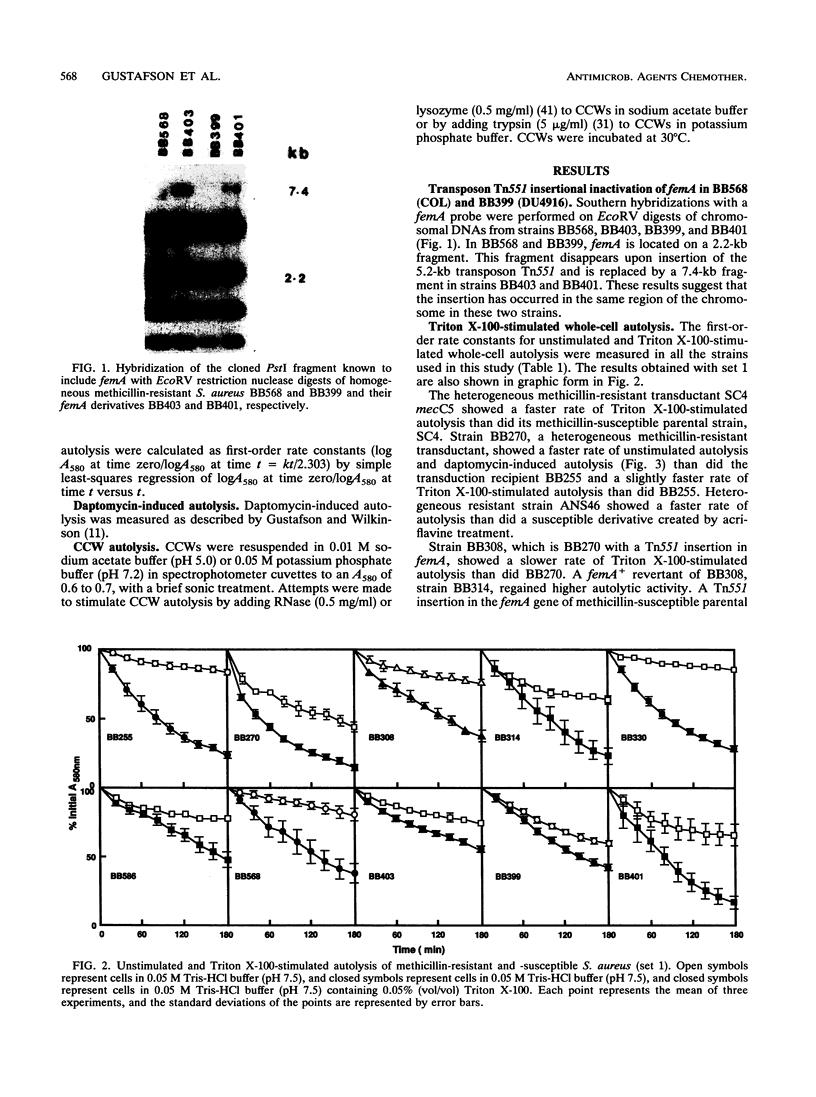
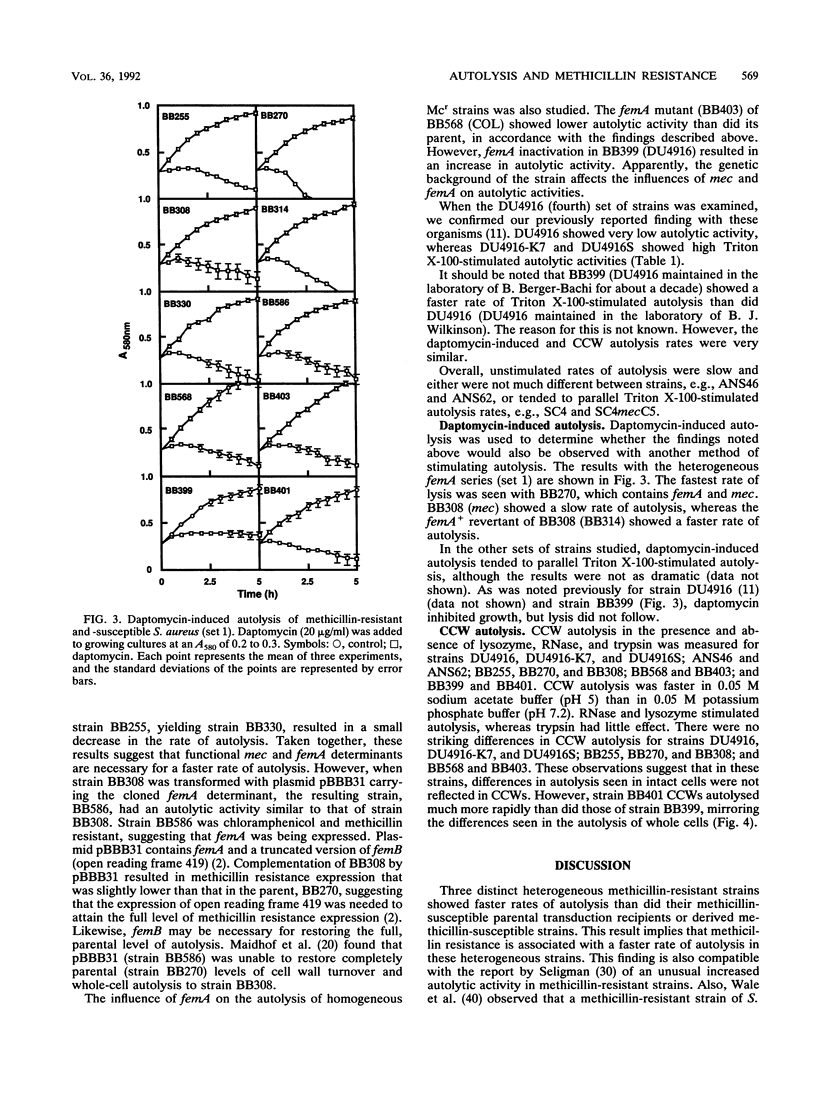
The autolytic activities, including unstimulated, Triton X-100-stimulated, and daptomycin-induced, of various sets of methicillin-resistant and related methicillin-susceptible strains were compared. Faster rates of autolysis were noted in two heterogeneous methicillin-resistant transductants than in their methicillin-susceptible parental recipients, in a heterogeneous resistant strain than in a susceptible derivative created by chemical mutagenesis, and in a homogeneous resistant strain than in a derivative that had decreased methicillin resistance and was created by transposon Tn551 mutagenesis. These results suggest that the presence of the methicillin resistance region, mec, either directly or indirectly through an interaction with other host genes, confers a faster rate of autolysis on strains. Various auxilliary genes are known to affect methicillin resistance expression, and one of these genes, femA, was necessary for the expression of this faster rate of autolysis. These differences in autolytic activities were not observed in isolated crude cell walls retaining autolytic activities, suggesting different modes of regulation of autolysins in intact cells and isolated walls. In contrast, one homogeneous, highly resistant strain, DU4916, had a lower autolytic activity than did derived heterogeneous resistant and susceptible strains created by chemical mutagenesis and a strain that had decreased resistance and was created by transposon mutagenesis. Our observations suggest that methicillin resistance expression is associated with an enhanced rate of autolysis, in heterogeneous resistant strains at least.
Full text
PDF
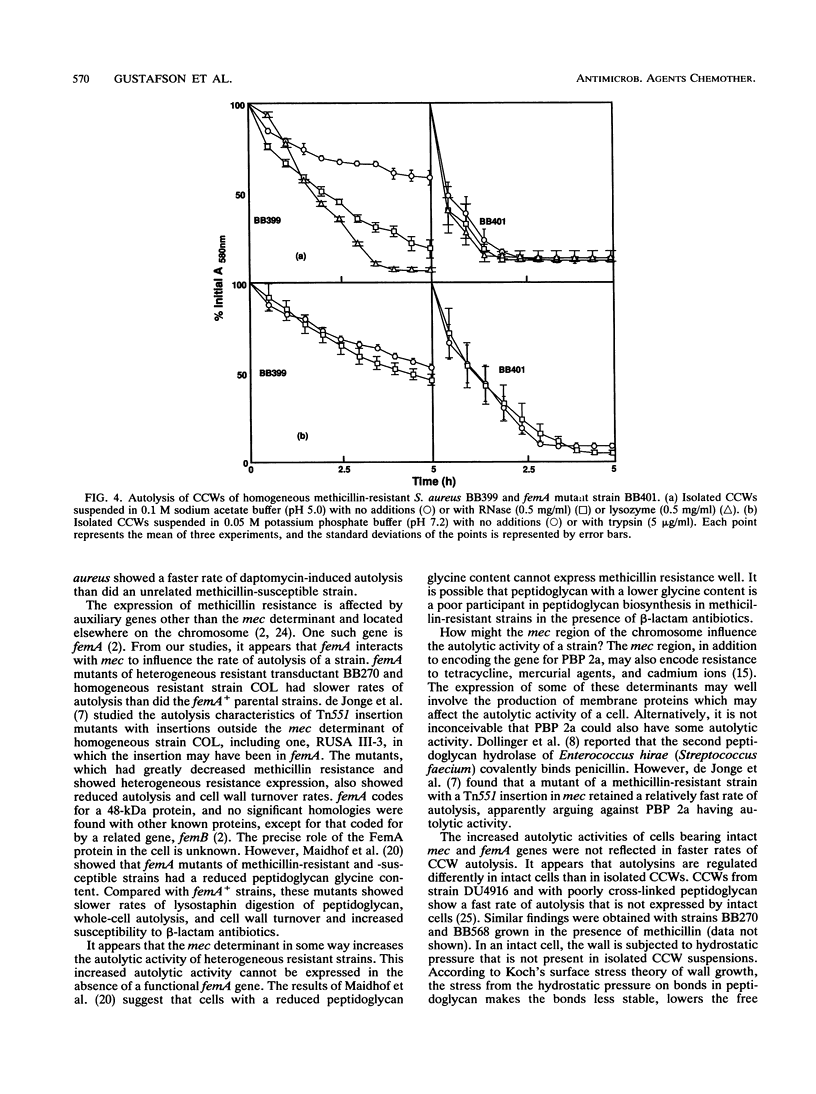


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger-Bächi B., Barberis-Maino L., Strässle A., Kayser F. H. FemA, a host-mediated factor essential for methicillin resistance in Staphylococcus aureus: molecular cloning and characterization. Mol Gen Genet. 1989 Oct;219(1-2):263–269. doi: 10.1007/BF00261186. [DOI] [PubMed] [Google Scholar]
- Berger-Bächi B. Insertional inactivation of staphylococcal methicillin resistance by Tn551. J Bacteriol. 1983 Apr;154(1):479–487. doi: 10.1128/jb.154.1.479-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Bächi B., Strässle A., Kayser F. H. Characterization of an isogenic set of methicillin-resistant and susceptible mutants of Staphylococcus aureus. Eur J Clin Microbiol. 1986 Dec;5(6):697–701. doi: 10.1007/BF02013308. [DOI] [PubMed] [Google Scholar]
- Chambers H. F., Hartman B. J., Tomasz A. Increased amounts of a novel penicillin-binding protein in a strain of methicillin-resistant Staphylococcus aureus exposed to nafcillin. J Clin Invest. 1985 Jul;76(1):325–331. doi: 10.1172/JCI111965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Transduction of Methicillin Resistance in Staphylococcus aureus Dependent on an Unusual Specificity of the Recipient Strain. J Bacteriol. 1970 Dec;104(3):1158–1167. doi: 10.1128/jb.104.3.1158-1167.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinger D. L., Daneo-Moore L., Shockman G. D. The second peptidoglycan hydrolase of Streptococcus faecium ATCC 9790 covalently binds penicillin. J Bacteriol. 1989 Aug;171(8):4355–4361. doi: 10.1128/jb.171.8.4355-4361.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch K., Hallander H. O., Löfquist F. Extrachromosomal control of methicillin resistance and toxin production in Staphylococcus aureus. J Bacteriol. 1969 May;98(2):351–358. doi: 10.1128/jb.98.2.351-358.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch K., Hallander H. O. Transduction of penicillinase production and methicillin resistance-enterotoxin B production in strains of Staphylococcus aureus. J Gen Microbiol. 1973 May;76(1):1–11. doi: 10.1099/00221287-76-1-1. [DOI] [PubMed] [Google Scholar]
- Gustafson J. E., Wilkinson B. J. Lower autolytic activity in a homogeneous methicillin-resistant Staphylococcus aureus strain compared to derived heterogeneous-resistant and susceptible strains. FEMS Microbiol Lett. 1989 May;50(1-2):107–111. doi: 10.1016/0378-1097(89)90468-0. [DOI] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Jan;29(1):85–92. doi: 10.1128/aac.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneine N., Stewart P. R. Physiological determination of methicillin resistance in Staphylococcus aureus: comparison of clinical and genetically derived isolates. J Antimicrob Chemother. 1986 Jun;17(6):705–715. doi: 10.1093/jac/17.6.705. [DOI] [PubMed] [Google Scholar]
- Inglis B., Matthews P. R., Stewart P. R. Induced deletions within a cluster of resistance genes in the mec region of the chromosome of Staphylococcus aureus. J Gen Microbiol. 1990 Nov;136(11):2231–2239. doi: 10.1099/00221287-136-11-2231. [DOI] [PubMed] [Google Scholar]
- Koch A. L. How bacteria grow and divide in spite of internal hydrostatic pressure. Can J Microbiol. 1985 Dec;31(12):1071–1084. doi: 10.1139/m85-204. [DOI] [PubMed] [Google Scholar]
- Kornblum J., Hartman B. J., Novick R. P., Tomasz A. Conversion of a homogeneously methicillin-resistant strain of Staphylococcus aureus to heterogeneous resistance by Tn551-mediated insertional inactivation. Eur J Clin Microbiol. 1986 Dec;5(6):714–718. doi: 10.1007/BF02013311. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Staphylococcus aureus strain DU4916--an atypical methicillin-resistant isolate? J Gen Microbiol. 1974 Sep;84(1):1–10. doi: 10.1099/00221287-84-1-1. [DOI] [PubMed] [Google Scholar]
- Madiraju M. V., Brunner D. P., Wilkinson B. J. Effects of temperature, NaCl, and methicillin on penicillin-binding proteins, growth, peptidoglycan synthesis, and autolysis in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1987 Nov;31(11):1727–1733. doi: 10.1128/aac.31.11.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidhof H., Reinicke B., Blümel P., Berger-Bächi B., Labischinski H. femA, which encodes a factor essential for expression of methicillin resistance, affects glycine content of peptidoglycan in methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. J Bacteriol. 1991 Jun;173(11):3507–3513. doi: 10.1128/jb.173.11.3507-3513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Song M. D., Ishino F., Wachi M., Doi M., Inoue M., Ubukata K., Yamashita N., Konno M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to beta-lactam antibiotics in Staphylococcus aureus. J Bacteriol. 1986 Sep;167(3):975–980. doi: 10.1128/jb.167.3.975-980.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. R., Reed K. C., Stewart P. R. The cloning of chromosomal DNA associated with methicillin and other resistances in Staphylococcus aureus. J Gen Microbiol. 1987 Jul;133(7):1919–1929. doi: 10.1099/00221287-133-7-1919. [DOI] [PubMed] [Google Scholar]
- Murakami K., Tomasz A. Involvement of multiple genetic determinants in high-level methicillin resistance in Staphylococcus aureus. J Bacteriol. 1989 Feb;171(2):874–879. doi: 10.1128/jb.171.2.874-879.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qoronfleh M. W., Wilkinson B. J. Effects of growth of methicillin-resistant and -susceptible Staphylococcus aureus in the presence of beta-lactams on peptidoglycan structure and susceptibility to lytic enzymes. Antimicrob Agents Chemother. 1986 Feb;29(2):250–257. doi: 10.1128/aac.29.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. E., Brown D. F. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. Effect of growth conditions. FEBS Lett. 1985 Nov 11;192(1):28–32. doi: 10.1016/0014-5793(85)80036-3. [DOI] [PubMed] [Google Scholar]
- Reynolds P. E. Resistance of the antibiotic target site. Br Med Bull. 1984 Jan;40(1):3–10. doi: 10.1093/oxfordjournals.bmb.a071944. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND R., ROLINSON G. N. CHARACTERISTICS OF METHICILLIN-RESISTANT STAPHYLOCOCCI. J Bacteriol. 1964 Apr;87:887–899. doi: 10.1128/jb.87.4.887-899.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D. Chemical and physical factors influencing methicillin resistance of Staphylococcus aureus and Staphylococcus epidermidis. J Antimicrob Chemother. 1977 Nov;3 (Suppl 100):47–51. doi: 10.1093/jac/3.suppl_c.47. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Smith P. F., Wilkinson B. J. Differential methicillin susceptibilities of peptidoglycan syntheses in methicillin-resistant Staphylococcus aureus. J Bacteriol. 1981 Nov;148(2):610–617. doi: 10.1128/jb.148.2.610-617.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M. D., Wachi M., Doi M., Ishino F., Matsuhashi M. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 1987 Aug 31;221(1):167–171. doi: 10.1016/0014-5793(87)80373-3. [DOI] [PubMed] [Google Scholar]
- Tesch W., Ryffel C., Strässle A., Kayser F. H., Berger-Bächi B. Evidence of a novel staphylococcal mec-encoded element (mecR) controlling expression of penicillin-binding protein 2'. Antimicrob Agents Chemother. 1990 Sep;34(9):1703–1706. doi: 10.1128/aac.34.9.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Nachman S., Leaf H. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob Agents Chemother. 1991 Jan;35(1):124–129. doi: 10.1128/aac.35.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wale L. J., Shelton A. P., Greenwood D. Scanning electronmicroscopy of Staphylococcus aureus and Enterococcus faecalis exposed to daptomycin. J Med Microbiol. 1989 Sep;30(1):45–49. doi: 10.1099/00222615-30-1-45. [DOI] [PubMed] [Google Scholar]
- Wecke J., Lahav M., Ginsburg I., Giesbrecht P. Cell wall degradation of Staphylococcus aureus by lysozyme. Arch Microbiol. 1982 Mar;131(2):116–123. doi: 10.1007/BF01053992. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. J., Dorian K. J., Sabath L. D. Cell wall composition and associated properties of methicillin-resistant Staphylococcus aureus strains. J Bacteriol. 1978 Dec;136(3):976–982. doi: 10.1128/jb.136.3.976-982.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Nadakavukaren M. J. Methicillin-resistant septal peptidoglycan synthesis in a methicillin-resistant Staphylococcus aureus strain. Antimicrob Agents Chemother. 1983 Apr;23(4):610–613. doi: 10.1128/aac.23.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Tomasz A. Inhibition of cell wall synthesis and acylation of the penicillin binding proteins during prolonged exposure of growing Streptococcus pneumoniae to benzylpenicillin. Eur J Biochem. 1985 Sep 16;151(3):475–483. doi: 10.1111/j.1432-1033.1985.tb09126.x. [DOI] [PubMed] [Google Scholar]
- Wyke A. W., Ward J. B., Hayes M. V. Synthesis of peptidoglycan in vivo in methicillin-resistant Staphylococcus aureus. Eur J Biochem. 1982 Oct;127(3):553–558. doi: 10.1111/j.1432-1033.1982.tb06907.x. [DOI] [PubMed] [Google Scholar]
- de Jonge B. L., de Lencastre H., Tomasz A. Suppression of autolysis and cell wall turnover in heterogeneous Tn551 mutants of a methicillin-resistant Staphylococcus aureus strain. J Bacteriol. 1991 Feb;173(3):1105–1110. doi: 10.1128/jb.173.3.1105-1110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]