Abstract
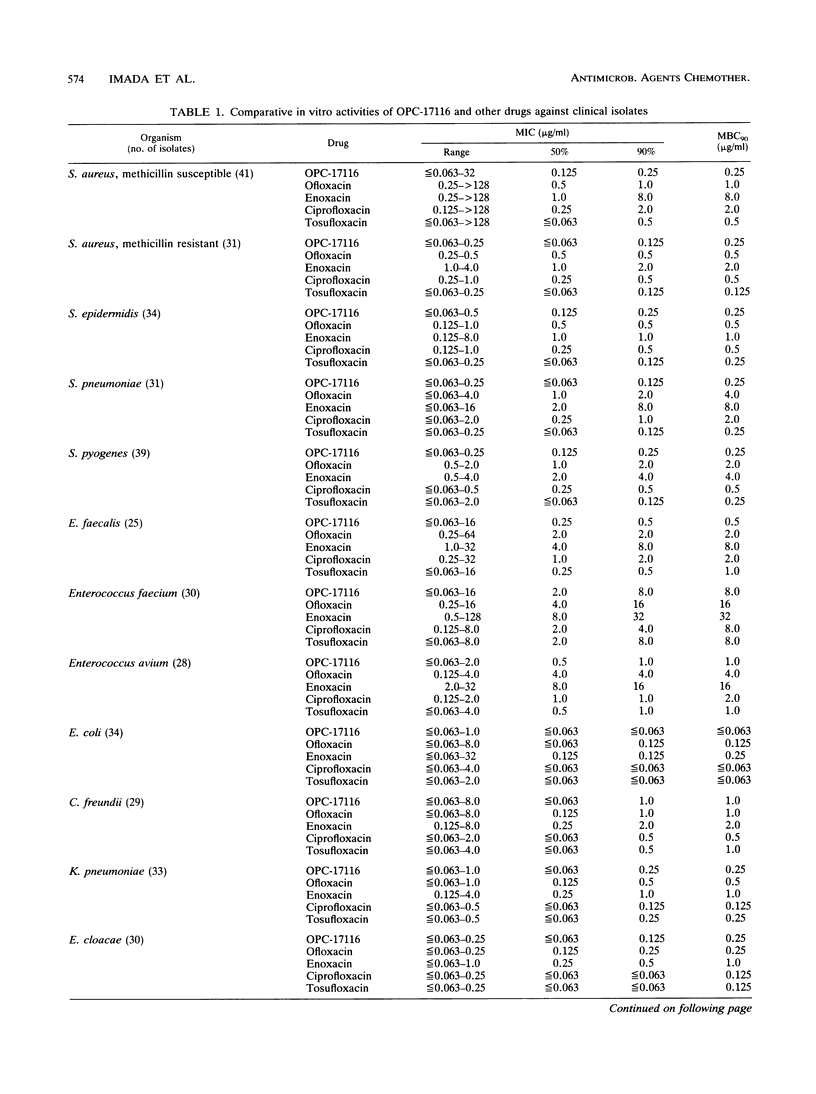
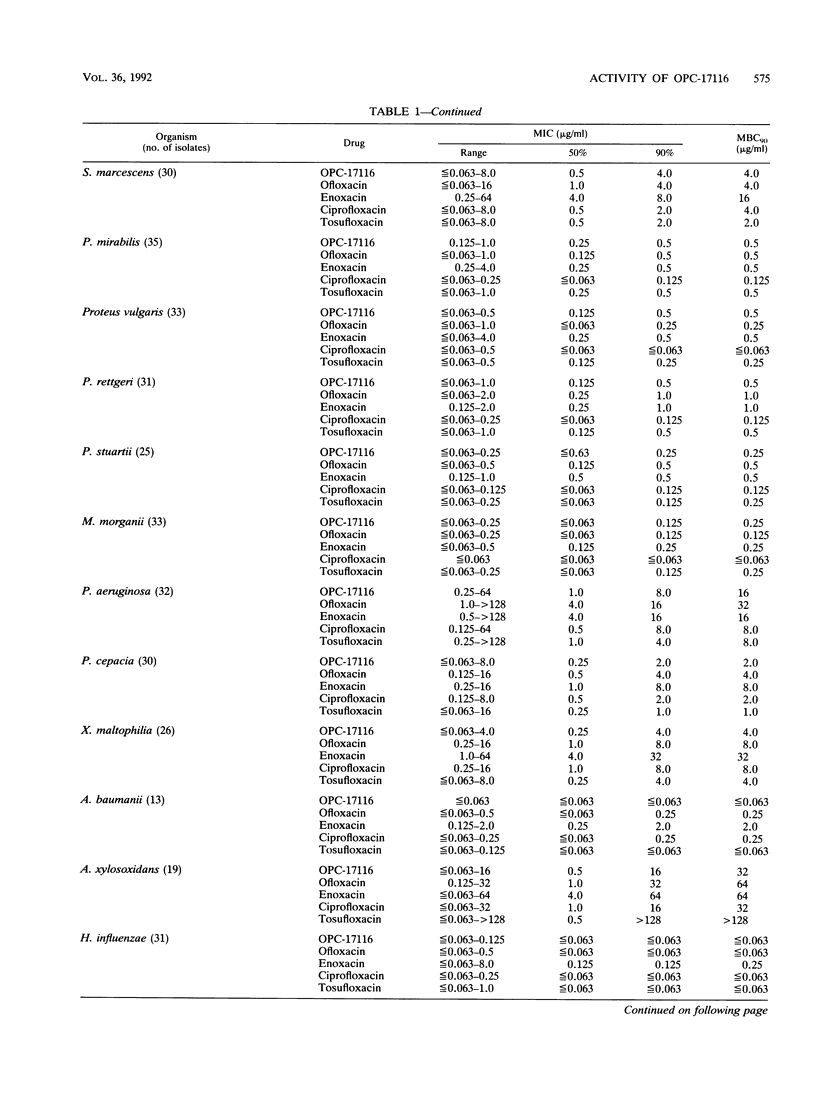
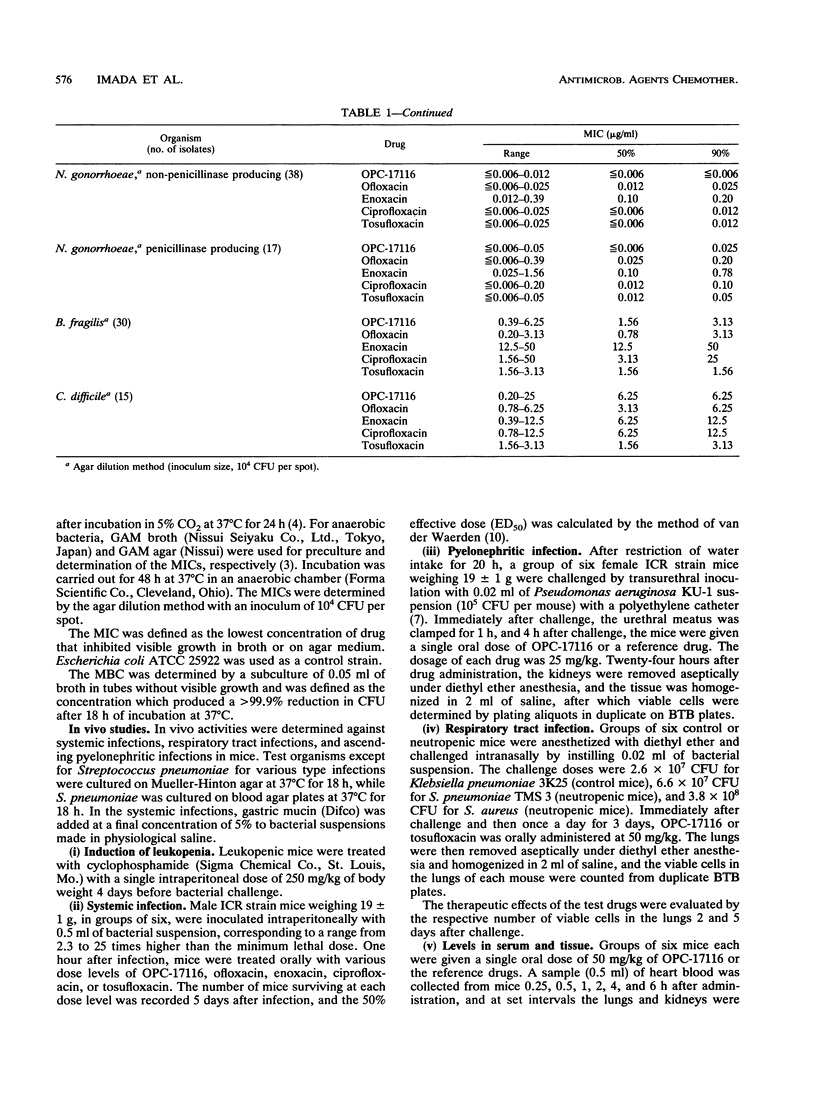
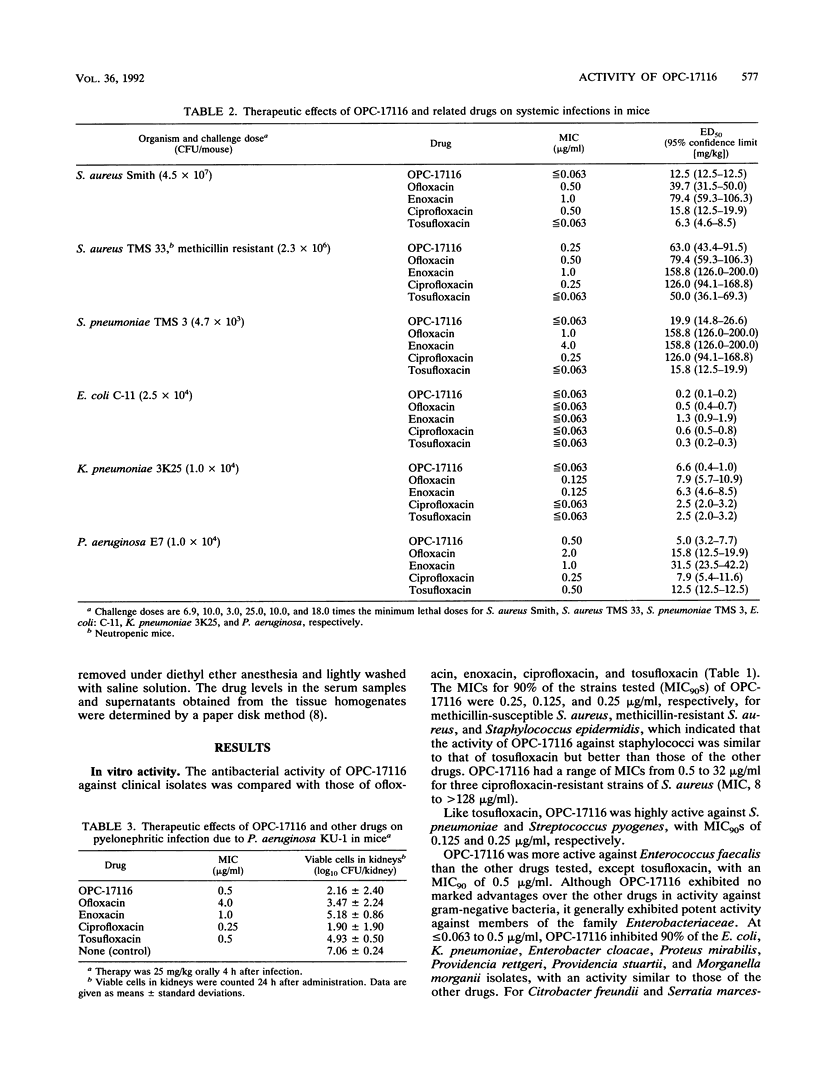
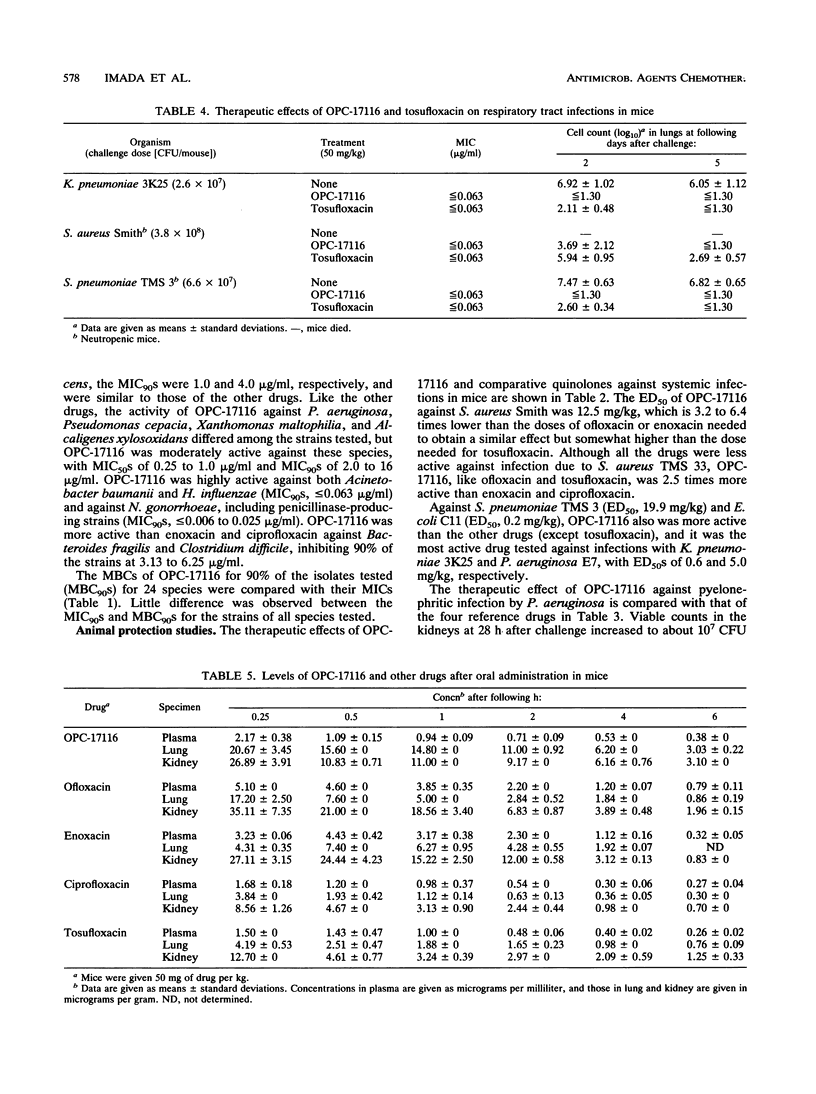
The in vitro and in vivo antibacterial activities of OPC-17116 were compared with those of ofloxacin, enoxacin, ciprofloxacin, and tosufloxacin. The MICs of OPC-17116 for 90% of the strains tested were 0.125 to 8 micrograms/ml against gram-positive bacteria such as members of the genera Staphylococcus, Streptococcus, and Enterococcus: less than or equal to 0.063 to 16 micrograms/ml against members of the family Enterobacteriaceae; and less than or equal to 0.063 to 16 micrograms/ml against glucose-nonfermentative bacilli such as Pseudomonas aeruginosa. The activity of OPC-17116 against gram-positive organisms was comparable to that of tosufloxacin and higher than those of other reference drugs. The in vitro activity of OPC-17116 against gram-negative bacteria was similar to those of the reference drugs. In experimental systemic infections in mice with various organisms, the efficacy of OPC-17116 was similar to that of tosufloxacin and greater than those of ofloxacin, enoxacin, and ciprofloxacin. In a pyelonephritic model in mice with P. aeruginosa KU-1, OPC-17116 was as active as ciprofloxacin and more active than ofloxacin, enoxacin, and tosufloxacin. In respiratory tract infections in mice with Staphylococcus aureus Smith, Streptococcus pneumoniae TMS 3, and Klebsiella pneumoniae 3K25, the efficacy of OPC-17116 was generally greater than that of tosufloxacin. The peak level of OPC-17116 in the lungs of mice was 10 times higher than that in serum and was significantly greater than levels in lung achieved with an equivalent dose of the other quinolones. The therapeutic efficacy of OPC-17116 may depend not only on its in vitro activity but also on its high concentration in tissue.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eliopoulos G. M., Gardella A., Moellering R. C., Jr In vitro activity of ciprofloxacin, a new carboxyquinoline antimicrobial agent. Antimicrob Agents Chemother. 1984 Mar;25(3):331–335. doi: 10.1128/aac.25.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimaki K., Noumi T., Saikawa I., Inoue M., Mitsuhashi S. In vitro and in vivo antibacterial activities of T-3262, a new fluoroquinolone. Antimicrob Agents Chemother. 1988 Jun;32(6):827–833. doi: 10.1128/aac.32.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouno K., Inoue M., Mitsuhashi S. In vitro and in vivo antibacterial activity of AT-2266. Antimicrob Agents Chemother. 1983 Jul;24(1):78–84. doi: 10.1128/aac.24.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi T., Tsuchiya K. Experimental urinary tract infection with Pseudomonas aeruginosa in mice. Infect Immun. 1978 Nov;22(2):508–515. doi: 10.1128/iai.22.2.508-515.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkin D. H., Sheikh W., Nadler H. Development of a meropenem susceptibility disc: drug content, preliminary interpretive and quality control criteria and potency bioassay. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):251–258. doi: 10.1093/jac/24.suppl_a.251. [DOI] [PubMed] [Google Scholar]
- Sato K., Matsuura Y., Inoue M., Une T., Osada Y., Ogawa H., Mitsuhashi S. In vitro and in vivo activity of DL-8280, a new oxazine derivative. Antimicrob Agents Chemother. 1982 Oct;22(4):548–553. doi: 10.1128/aac.22.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiler H. J., Grohe K. The in vitro and in vivo activity of ciprofloxacin. Eur J Clin Microbiol. 1984 Aug;3(4):339–343. doi: 10.1007/BF01977490. [DOI] [PubMed] [Google Scholar]


