Abstract
MS is a demyelinating disease characterized by infiltration of monocytes and lymphocytes into the brain parenchyma, destruction of oligodendrocytes and loss of myelin. Since chemokines play a major role in the migration of monocytes and T cells, we here investigated the expression of the CC chemokines MIP-1α, MIP-1β, and RANTES in brain tissue from MS patients using reverse transcriptase-polymerase chain reaction techniques. Both MIP-1β as well as RANTES were found to be significantly elevated in brain tissue of MS patients. In addition, MIP-1α was also increased, although not significantly. Immunohistochemistry revealed that, whereas RANTES was mainly localized in reactive astrocytes, MIP-1α and MIP-1β immunoreactivity was predominantly found in perivascular and parenchymal macrophages, containing myelin degradation products. Thus, chemokines appear to be associated with MS and an increased chemokine expression may further enhance disease progression by attracting more leucocytes into the brain parenchyma and by activation of effector functions of astrocytes and microglial cells.
Keywords: CC chemokines, macrophages, astrocytes, reverse transcriptase-polymerase chain reaction, immunohistochemistry
Introduction
Chemokines are low molecular weight molecules (8–10 kD) that are able to attract various cell types to sites of infection and inflammation. They play an important role in this chemoattraction and subsequent activation of these cells by binding to specific cell-surface receptors that belong to a superfamily of G protein-coupled seven-transmembrane-segment receptors [1]. Two major chemokine families, the CC and the CXC chemokines, can be distinguished based on their function, sequence, and chromosomal location. CC chemokines preferentially attract monocytes and lymphocytes, whereas CXC chemokines mainly have a chemotactic effect on neutrophils.
The CC chemokines, that are associated with chronic inflammation, have been implicated in diseases characterized by monocyte-rich infiltrates, such as atherosclerosis [2], rheumatoid arthritis [3], AIDS dementia complex [4,5] and might also participate in the pathogenesis of MS [6,7]. MS is a chronic demyelinating disease of the central nervous system (CNS), characterized by neurological symptoms caused by impaired nerve conduction. Clinical signs of MS are a result of inflammatory lesions in the CNS. Early in lesion development there is breakdown of the blood–brain barrier (BBB), allowing mononuclear cells to enter the CNS. Neuropathology of MS is characterized by an inflammatory perivascular infiltrate, predominately consisting of macrophages, a large number of parenchymal macrophages, astrocytic gliosis and loss of myelin in the white matter [8]. Macrophages are believed to play an active part in demyelinization by stripping of myelin lamellae [9] and phagocytosis of the myelin proteins [10]. Since the recruitment of blood monocytes into the brain parenchyma appears to be correlated with disease progression, mechanisms of this recruitment are currently studied intensively. Chemokine expression, and in particular monocyte chemoattractant protein-1 (MCP-1), has been studied in demyelinating MS lesions by various groups using immunohistochemical techniques [7,11,12] and chemokine levels have been measured in the cerebrospinal fluid (CSF) of MS patients [13]. In addition, McManus et al. have demonstrated MCP-1 mRNA expression by reactive astrocytes and inflammatory macrophages using in situ hybridization techniques [7]. However, this is the first study that compares mRNA expression levels of the CC chemokines, MIP-1α, MIP-1β, and regulated upon activation, normal T cell expressed and secreted (RANTES), in post mortem brain tissue of MS cases with chemokine mRNA expression levels in brain tissue of normal cases. Furthermore, we performed immunohistochemical staining on frozen tissue sections derived from actively demyelinating MS lesions to determine the cellular localization of different CC chemokines.
Materials and methods
Human brain tissue samples
Human brain tissue was obtained at autopsy (with short post mortem intervals; see Table 1) from six MS cases and six age-matched cases without a history of brain disease. The autopsies were performed under the management of the Netherlands Brain Bank, Amsterdam (coordinator Dr R. Ravid). In all MS cases, multiple tissue samples were taken from lesions located in the brain. Tissue samples from non-neurological control cases were taken from the subcortical white matter or corpus callosum. Subsequently, for all samples, 10 serial sections were obtained for RNA isolation. The clinical diagnosis of MS was confirmed neuropathologically. Brain tissue samples were snap-frozen in liquid nitrogen and stored at −196°C. Haematoxylin and eosin (H–E)-stained sections were prepared from the obtained brain tissue. Tissue samples derived from MS lesions were stained with the neutral lipid marker oil red O (ORO) to delineate areas of myelin breakdown and demyelination, with KP1 (CD68) and LCA (CD45) to detect leucocyte infiltration, and with anti-glial fibrillary acidic protein (anti-GFAP) to determine the extent of astrogliosis (see below).
Table 1.
Details of MS and normal control autopsy brain tssue
| NBB* no. | Age, years | Sex | Post mortem delay |
|---|---|---|---|
| Multiple sclerosis cases: | |||
| 96-040 | 35 | F | 5 h 45 min |
| 96-074 | 40 | F | 7 h 00 min |
| 96-076 | 81 | F | 4 h 15 min |
| 96-121 | 53 | F | 7 h 15 min |
| 97-160 | 40 | F | 7 h 00 min |
| 97-050 | 85 | F | 4 h 00 min |
| Normal control cases: | |||
| 94-113 | 82 | F | 6 h 30 min |
| 94-119 | 51 | F | 7 h 45 min |
| 94-125 | 51 | M | 6 h 00 min |
| 95-007 | 54 | F | 9 h 15 min |
| 98-125 | 58 | F | 6 h 15 min |
| 98-127 | 56 | M | 5 h 30 min |
NBB, Netherlands brain bank.
Reverse transcriptase-polymerase chain reaction detection of chemokines
Brain tissue was homogenized and lysed in 1 ml TRIzol (Life Technologies, Gaithersburg, MD) according to the manufacturer's guidelines. Total RNA was isolated and dissolved in diethylpyrocarbonate (DEPC)-treated water and 1 μ g of RNA was used for the synthesis of complementary DNA and polymerase chain reactions (PCR) were performed as described previously [14]. Amplification of the cDNA was accomplished using one primer biotinylated on the 5′ terminal nucleotide to facilitate later capture using streptavidin. To confirm single band product positive reactions were subjected to 40 cycles amplification and electrophoresis followed by ethidium bromide staining. For semiquantification, all primer pairs were tested at different cycle numbers to determine the linear range. GAPDH mRNA levels were measured at 30 cycles, whereas cDNA had to be subjected to 32 cycles to be in the linear range to detect MIP-1α, MIP-1β, and 37 cycles for RANTES.
Aliquots of 5 μ l of the biotinylated PCR product were semiquantitatively analysed using a fluorescent digoxigenin (DIG) detection ELISA kit (Boehringer Mannheim, Germany) according to the manufacturer's protocol as described previously [14]. In short, the biotinylated strand of denatured PCR product was captured by immobilized streptavidin. Then, a DIG-labelled specific probe was added, followed by an alkaline phosphatase-labelled antibody against DIG. After addition of the substrate, fluorescence was measured in relative fluorescence units (RFU) in a fluorescence multiwell plate reader (Perseptive Biosystems, Framingham, MA) at excitation 450 nm/emission 550 nm. All data were normalized against GAPDH mRNA levels, which was used as an internal standard. Data were compared, and a Kruskal–Wallis H-test was used to determine P values. Primer and probe sequences are shown in Table 2.
Table 2.
Sequences of the oligonucleotide primers and probes in reverse transcriptase-polymerase chain reaction
| Target (product size) | Sequence 5′-3′ | |
|---|---|---|
| GAPDH | Sense | CCATGGAGAAGGCTGGGG |
| (195 bp) | Antisense | CAAAGTTGTCATGGATGACC |
| Probe | CTGCACCACCAACTGCTTAGC | |
| MIP-1α | Sense | TGCATCACTTGCTGCTGACACG |
| (333 bp) | Antisense | CAACCAGTCCATAGAAGAGG |
| Probe | CTGACTACTTTGAGACGAGC | |
| MIP-1β | Sense | CCAAACCAAAAGAAGCAAGC |
| (310 bp) | Antisense | AGAAACAGTGACAGTGGACC |
| Probe | ACATCTCCTCCATACTCAGG | |
| RANTES | Sense | CTTTGTCACCCGAAAGAACC |
| (352 bp) | Antisense | GTTTCATCATGTTGGCCAGG |
| Probe | TTGCTCTTGTCCTAGCTTGG |
Immunohistochemistry
Mouse anti-human MIP-1α (IgG2a), MIP-1β (IgG2b) and RANTES (IgG1) were obtained from R&D Systems (Abingdon, UK). Mouse anti-human KP1 (CD68; IgG1), mouse anti-human leucocyte common antigen (LCA; CD45; IgG1) and rabbit anti-cow GFAP were obtained from Dako (Glostrup, Denmark). Purified mouse myeloma protein IgG1 (κ), used as an isotype-specific control antibody, was obtained from ICN Pharmaceuticals (Aurora, OH).
Frozen sections (5 μ m thick) of MS lesions and normal control CNS tissue were mounted on poly L-lysine (PLL)-coated glass slides, air dried and fixed in acetone for 10 min at room temperature. All washes were carried out for 15 min with 0·01 m PBS pH 7·4 and antibodies were diluted in PBS containing 1% bovine serum albumin (BSA). To prevent aspecific binding, sections were preincubated with 10% normal swine serum (for polyclonal antibodies (pAbs)) or with 2% normal rabbit serum (for MoAbs) for 10 min at room temperature. Primary antibodies were diluted in PBS–BSA as follows: MIP-1α 1:10, MIP-1β 1:10, RANTES 1:50, KP1 1:400, LCA 1:50 and GFAP 1:1000, and incubated for 1 h at room temperature, followed by washing. Control sections were incubated with mouse purified IgG1 (1:100 dilution). After washing immunolabelling with primary antibodies was detected with biotinylated rabbit anti-mouse or biotinylated swine anti-rabbit (Dako) for 30 min at room temperature and avidin–biotin–peroxidase complexes (ABC; Vector Labs, Burlingame, CA) for 1 h at room temperature. Peroxidase activity was demonstrated with 0·5 mg/ml 3,3′-diaminobenzidine tetrachloride (DAB; Sigma, St Louis, MO) in 0·05 m Tris–HCl buffer pH 7·6 containing 0·03% H2O2. Sections were counterstained with haematoxylin, dehydrated and mounted in Entellan (Merck, Darmstadt, Germany).
All sections were evaluated by light microscopy, and the immunoreactivity was scored by assignment of −, +/−, + or ++ for no, weak, moderate, and strong immunoreactivity, respectively.
Results
Neuropathological evaluation
Frozen sections from all MS lesions were histochemically stained with ORO and immunohistochemically with KP1, LCA and GFAP antibodies to evaluate MS lesion activity. The selected MS lesions contained abundant phagocytic, ORO- and KP1-positive foamy macrophages (Fig. 1A,B) and were classified as active demyelinating. In normal control white matter (corpus callosum or subcortical white matter) no activity of inflammatory cells was detected. Reactive astrocytes immunoreactive for GFAP were distributed throughout the lesion (Fig. 1C).
Fig. 1.
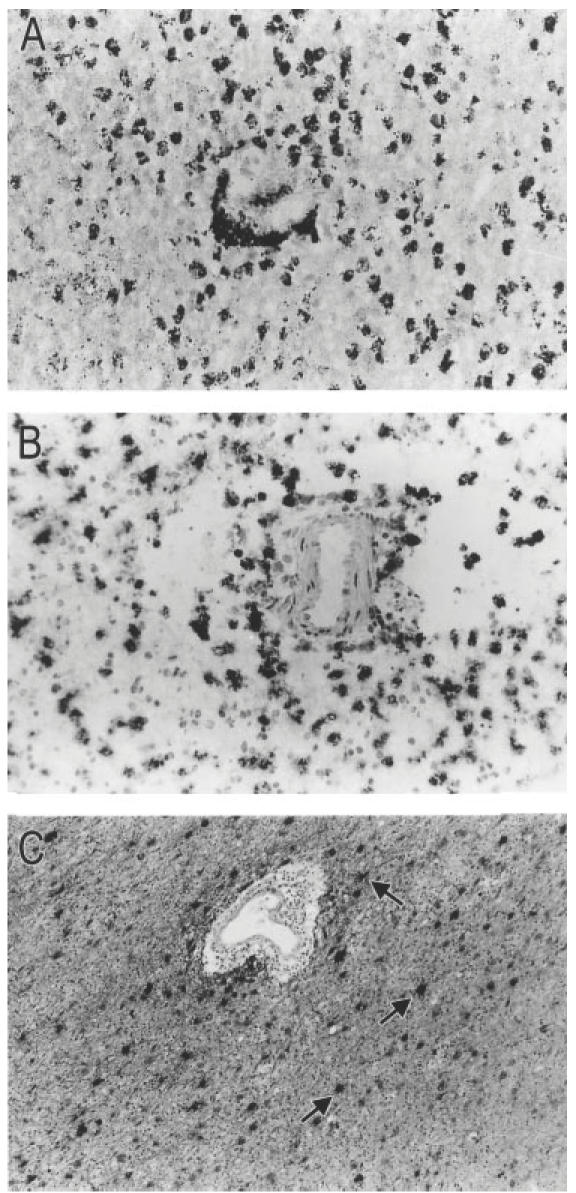
Frozen sections from active demyelinating MS brain lesions (case no. 96-040; 97-160). The sections have been counterstained with haematoxylin which stains nuclei blue. (A) Histochemical staining with the neutral lipid marker oil red O (ORO). Abundant myelin debris-filled ORO+ perivascular and parenchymal macrophages are distributed throughout the demyelinated lesion. (B) A serial section showing numerous phagocytic macrophages that are strongly immunoreactive for the macrophage-specific marker KP1 (CD68). (C) Reactive astrocytes are strongly immunoreactive for glial fibrillary acidic protein (GFAP, arrows). (Mag. × 180.)
MIP-1β and RANTES mRNA levels are significantly elevated in active demyelinating MS lesions
A semiquantitative fluorescence assay was used to study the levels of expression of the three CC chemokines MIP-1α, MIP-1β, and RANTES in post mortem brain tissue of MS cases and age-matched control cases (C). The mean mRNA levels after three PCR rounds of all gene products, expressed in RFU, are depicted in Fig. 2. MIP-1β and RANTES mRNA was detected in the MS group at significantly higher levels than in the control group (P < 0·05 and P < 0·01, respectively). RANTES mRNA was much less abundant than MIP-1β, as indicated by the number of PCR rounds needed for linear amplification. Furthermore, although MIP-1α levels were increased in brain tissue of the MS patients compared with the control patients, this increase was not significant (P < 0·2).
Fig. 2.
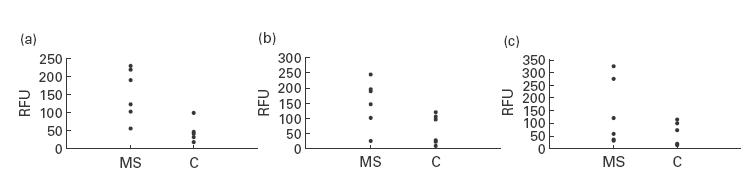
Chemokine mRNA levels in the frontal cortex of post mortem brain tissue of MS patients and age-matched control patients (C) expressed as relative fluorescence units (RFU). Significantly elevated gene expression for regulated upon activation, normal T cell expressed and secreted (RANTES) ((a), P < 0·05) and MIP-1β ((b), P < 0·05) was found in MS patients compared with control patients. MIP-1α mRNA levels were increased, although not significantly ((c), P > 0·05) in MS patients. P values were calculated using Kruskal-Wallis H-test. Results are representative of at least three independent polymerase chain reaction experiments.
Immunohistochemical staining for MIP-1α, MIP-1β and RANTES on normal control brain tissue and in active demyelinating MS lesions
In frozen tissue sections from the white matter derived from normal control cases no immunoreactivity for MIP-1β could be detected. RANTES was weakly expressed by astrocytes surrounding blood vessel walls (not shown) and MIP-1α was weakly expressed by resident microglia (arrows) throughout the white matter of control cases (Fig. 3).
Fig. 3.
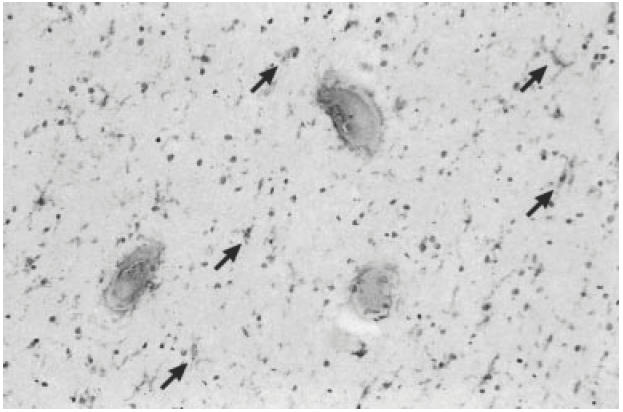
Frozen sections from the white matter of a normal control case (case no. 94-119). The sections have been counterstained with haematoxylin which stains nuclei blue. Immunohistochemical staining with a MIP-1α MoAb of the subcortical white matter showing immunoreactive resident microglia (arrows) distributed throughout the brain parenchyma. (Mag. × 180.)
In active demyelinating MS lesions a moderate staining for RANTES was detected in cells morphologically resembling reactive astrocytes (Fig. 4A). Adjacent normal appearing white matter (NAWM) demonstrated weak immunoreactivity for RANTES in reactive astrocytes. In a serial section astrocytes were identified by GFAP staining (Fig. 4B). Furthermore, neither macrophages and lymphocytes present in perivascular infiltrates, nor parenchymal phagocytic macrophages displayed RANTES immunoreactivity. With the IgG1 isotype-matched control antibody no aspecific staining was detected (not shown). A moderate immunoreactivity for MIP-1α was detected in myelin debris-containing perivascular and parenchymal macrophages distributed throughout the demyelinated region (Fig. 4C), whereas weak MIP-1α staining was found in microglia in NAWM. Strong MIP-1β immunoreactivity could be detected in phagocytic macrophages (Fig. 4D) in active demyelinating lesions. Staining on serial sections using KP1 (CD68) antibodies confirmed the phenotype of these cells (see Fig. 1B). Table 3 gives a summary of chemokine distribution in normal control brain tissue, active demyelinating MS lesions and adjacent NAWM. This Table shows representative results from three areas for each sample, for all cases.
Fig. 4.
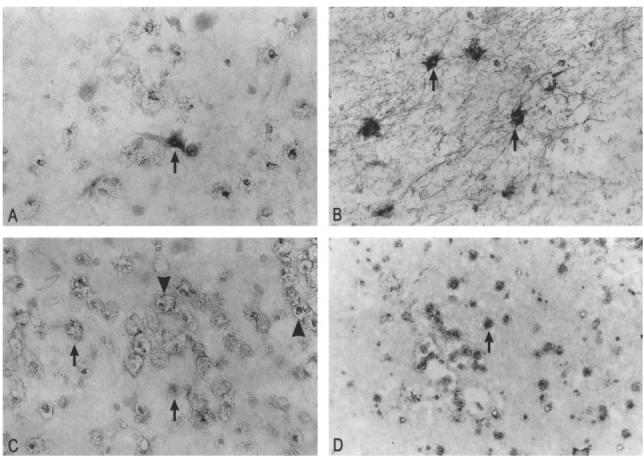
Frozen sections from active demyelinating MS lesions (case no. 96-040; 97-160). The sections have been counterstained with haematoxylin which stains nuclei blue. (A) Immunohistochemical staining with a regulated upon activation, normal T cell expressed and secreted (RANTES) MoAb of an active demyelinating MS lesion. A moderate immunoreactivity for RANTES was detected in virtually all reactive astrocytes (arrows). (B) In a serial section, reactive astrocytes in active demyelinating lesions demonstrate strong immunoreactivity for glial fibrillary acidic protein (GFAP) (arrows). (C) Immunohistochemical staining with a MIP-1α MoAb of an active demyelinating MS lesion. A moderate immunoreactivity for MIP-1α was detected in myelin debris-containing perivascular macrophages (arrowhead) and parenchymal macrophages (arrows) distributed throughout the lesion. (D) On a serial section a strong immunoreactivity was detected for MIP-1β in these foamy macrophages (arrows). (Mag. × 180 (D) and ×360 (A,B,C.)
Table 3.
Immunohistochemical staining patterns of chemokine expression in normal control brain tissue and active demyelinating lesions and adjacent normal appearing white matter (NAWM)
| Resident microglia | (Reactive)astrocytes | |||||
|---|---|---|---|---|---|---|
| Antibody | Control* | NAWM† | Phagocytic marcrophages MS lesion‡ | Control | NAWM | MS lesion |
| RANTES | − | – | − | +/− | +/− | + |
| MIP-1α | +/– | +/ | + | – | – | – |
| MIP-1β | – | – | ++ | − | − | − |
−, No staining; +/−, weak staining; +, moderate staining; ++, strong staining.
Control: brain tissue of non-neurological control cases.
NAWM: normal appearing white matter of MS brains.
MS lesion: active demyelinating lesions.
Discussion
Although both chronic and active demyelinating MS lesions contain far more macrophages than T cells, the role of macrophages in MS is still not completely understood. Macrophages have been associated with neurotoxic as well as neurotrophic mechanisms in MS [15] and are therefore probably important regulators of brain homeostasis. Macrophage-associated neurotoxicity is also found in neurodegenerative diseases that are characterized by macrophage infiltration, like AIDS dementia complex (ADC). In brain tissue of patients suffering from ADC a variety of macrophage-derived neurotoxins can be detected [16] and immune activation may play a pivotal role in disease progression [14,17]. Similarly, in MS patients mononuclear cells have been shown to secrete proinflammatory cytokines [8,18] which are also likely to exert detrimental effects. In experimental allergic encephalomyelitis (EAE), a frequently studied animal model system for demyelination, it was shown that activated macrophages secrete molecules that play a role in the development of the clinical and pathological expression of EAE and depletion of macrophages even prevented EAE [19,20]. Another major pathophysiological mechanism may involve an elevated secretion of matrix metalloproteinases (MMPs) by macrophages. MMPs are proteinases that may be involved in various mechanisms leading to tissue destruction and inflammation like degradation of myelin, breakdown of the blood–brain barrier and subsequent leucocyte migration into the CNS, and immune activation [21,22]. However, brain macrophages also appear to have beneficial effects [23]. Cytokine signalling may result in the induction of growth factors that may promote proliferation of oligodendrocytes and myelin regeneration [24,25]. In fact, in EAE it was shown that insulin growth factor 1 can reduce lesion severity and promote myelin regeneration, and neurotrophic factors are being considered as potential therapy for MS [26,27].
Thus, macrophages appear to play a determining role in the progression of MS and it is essential that mechanisms of leucocyte recruitment are studied. The results of this study strengthen the current hypothesis that chemokines may be involved in lesion formation by attracting lymphocytes and monocytes into the brain parenchyma. Moreover, in addition to the recruitment of leucocytes into a site of tissue damage, CC chemokines are involved in the activation of effector functions of leucocytes. MCP-1 stimulates release of lysosomal enzymes and the respiratory burst in monocytes [28]. It is also thought that CC chemokines, like RANTES and MIP-1α, provide a necessary signal for T lymphocyte activation [29,30], thereby facilitating antigen presentation. Recently it was shown that, besides chemokines, chemokine receptor expression may also be modulated in different stages of MS [31,32], which suggests that differential chemokine receptor expression may be involved in disease development. Balashov et al. also suggest an important role for MIP-1α, but do not study MIP-1β and RANTES, nor do they quantify expression [31]. In the present study, it is shown that brain tissue of MS patients demonstrates a significant increase of MIP-1β and RANTES mRNA expression. MIP-1α mRNA expression levels were not significantly increased, but were elevated, which corresponds with the protein expression detected in normal control brain tissue and in MS lesions. Considering the increase in CCR5-positive cells [31,32], which is the receptor for RANTES, MIP-1α and MIP-1β, this increase in chemokine expression is very likely to have major consequences. By immunohistochemical analysis of serial post mortem brain tissue sections we showed that both reactive astrocytes as well as phagocytic perivascular and parenchymal macrophages and locally activated microglial cells are involved in the production of chemokines. Although the results of the mRNA semiquantification are in agreement with previous studies [6,13,32], the localization varies. Whereas Hvas and colleagues demonstrated RANTES immunoreactivity primarily in T cells, we and others show RANTES staining in reactive astrocytes [11], which is strengthened by an in vitro study that shows that local inflammation can induce RANTES in astrocytes [33]. There are also discrepancies in the localization of other chemokines, although MIP-1β appears to be only associated with macrophages [11,34]. These differences in the determination of the cellular source of chemokines may be caused by suboptimal staining techniques and different expression levels.
Thus, in addition to focusing therapeutic strategies on the inhibition of pathophysiological mechanisms or on the enhancement of neurotrophic mechanism, preventing massive infiltration of macrophages into the brain parenchyma and inhibition of macrophage effector functions may also provide a successful strategy against MS. Blocking actions of molecules like chemokines that enhance macrophage infiltration will therefore be an important goal of future studies.
Acknowledgments
The authors would like to thank the Netherlands Brain Bank for supplying the human CNS tissue (coordinator Dr R. Ravid) and Dr W. Kamphorst for the neuropathological evaluation. We are also grateful to Jaap van Veldhuisen and Hans Oskam for preparing the illustrations. H.S.L.M.N. is a fellow of the Royal Netherlands Academy of Sciences and Arts. This study was partly supported by a grant from the Dutch Foundation ‘Vrienden MS Research’ (95-237).
References
- 1.Horuk R. Molecular properties of the chemokine receptor family. Trends Pharmacol Sci. 1994;15:159–65. doi: 10.1016/0165-6147(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 2.Takeya M. Detection of monocyte chemoattractant protein-1 in human atherosclerotic lesions by an anti-monocyte chemoattractant protein-1 monoclonal antibody. Hum Pathol. 1993;24:534–9. doi: 10.1016/0046-8177(93)90166-e. [DOI] [PubMed] [Google Scholar]
- 3.Strieter RM, Koch AE, Antony VB, Fick Rb, Jr, Standiford TJ, Kunkel SL. The immunopathology of chemotactic cytokines: the role of interleukin-8 and monocyte chemoattractant protein-1. J Lab Clin Med. 1994;123:183–97. [PubMed] [Google Scholar]
- 4.Conant K, Garzino Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–21. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidtmayerova H, Nottet HS, Nuovo G, et al. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci USA. 1996;93:700–4. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hvas J, McLean C, Justesen J, Kannourakis G, Steinman L, Oksenberg JR, Bernard CC. Perivascular T cells express the pro-inflammatory chemokine RANTES mRNA in multiple sclerosis lesions. Scand J Immunol. 1997;46:195–203. doi: 10.1046/j.1365-3083.1997.d01-100.x. [DOI] [PubMed] [Google Scholar]
- 7.McManus C, Berman JW, Brett FM, Staunton H, Farrell M, Brosnan CF. MCP-1, MCP-2 and MCP-3 expression in multiple sclerosis lesions: an immunohistochemical and in situ hybridization study. J Neuroimmunol. 1998;86:20–29. doi: 10.1016/s0165-5728(98)00002-2. [DOI] [PubMed] [Google Scholar]
- 8.Al-Omaishi J, Bashir R, Gendelman HE. The cellular immunology of multiple sclerosis. J Leuk Biol. 1999;65:444–52. doi: 10.1002/jlb.65.4.444. [DOI] [PubMed] [Google Scholar]
- 9.Brosnan CF, Raine CS. Mechanisms of immune injury in multiple sclerosis. Brain Pathol. 1996;6:243–57. doi: 10.1111/j.1750-3639.1996.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 10.Bauer J, Sminia T, Wouterlood FG, Dijkstra CD. Phagocytic activity of macrophages and microglial cells during acute and chronic relapsing experimental autoimmune encephalomyelitis. J Neurosci Res. 1994;38:365–75. doi: 10.1002/jnr.490380402. [DOI] [PubMed] [Google Scholar]
- 11.Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN. Expression of monocyte chemoattractant protein-1 and other beta-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J Neuroimmunol. 1998;84:238–49. doi: 10.1016/s0165-5728(97)00208-7. [DOI] [PubMed] [Google Scholar]
- 12.Van Der Voorn P, Tekstra J, Beelen RHJ, Tensen CP, Van der Valk P, de Groot CJ. Expression of MCP-1 by reactive astrocytes in demyelinating multiple sclerosis lesions. Am J Pathol. 1999;154:45–51. doi: 10.1016/S0002-9440(10)65249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyagishi R, Kikuchi S, Fukazawa T, Tashiro K. Macrophage inflammatory protein-1 alpha in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological diseases. J Neurol Sci. 1995;129:223–7. doi: 10.1016/0022-510x(95)00004-l. [DOI] [PubMed] [Google Scholar]
- 14.Boven LA, Gomes L, Hery C, Gray F, Verhoef J, Portegies P, Tardieu M, Nottet HSLM. Increased peroxynitrite activity in AIDS Dementia Complex: implications for the neuropathogenesis of HIV-1 infection. J Immunol. 1999;162:4319–27. [PubMed] [Google Scholar]
- 15.Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997;75:165–73. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- 16.Nottet HS, Bar DR, van Hassel H, Verhoef J, Boven LA. Cellular aspects of HIV-1 infection of macrophages leading to neuronal dysfunction in in vitro models for HIV-1 encephalitis. J Leuk Biol. 1997;62:107–16. doi: 10.1002/jlb.62.1.107. [DOI] [PubMed] [Google Scholar]
- 17.Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–60. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- 18.Sarchielli P, Orlacchio A, Vicinanza F, Pelliccioli G, Tognoloni M, Saccardi C, Gallai V. Cytokine secretion and nitric oxide production by mononuclear cells of patients with multiple sclerosis. J Neuroimmunol. 1997;80:76–86. doi: 10.1016/s0165-5728(97)00136-7. [DOI] [PubMed] [Google Scholar]
- 19.Brosnan CF, Bornstein MB, Bloom BR. The effects of macrophage depletion on the clinical and pathologic expression of experimental allergic encephalomyelitis. J Immunol. 1999;126:614–20. [PubMed] [Google Scholar]
- 20.Huitinga I, van Rooijen N, de Groot CJ, Uitdehaag BM, Dijkstra CD. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med. 1990;172:1025–33. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cossins JA, Clements JM, Ford J, Miller KM, Pigott R, Vos W, Van der Valk P, de Groot CJ. Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta Neuropathol Berl. 1997;94:590–8. doi: 10.1007/s004010050754. [DOI] [PubMed] [Google Scholar]
- 22.Maeda A, Sobel RA. Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropathol Exp Neurol. 1996;55:300–9. doi: 10.1097/00005072-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Mallat M, Chamak B. Brain macrophages: neurotoxic or neurotrophic effector cells? J Leuk Biol. 1994;56:416–22. doi: 10.1002/jlb.56.3.416. [DOI] [PubMed] [Google Scholar]
- 24.Bansal R, Pfeiffer SE. Regulation of oligodendrocyte differentiation by fibroblast growth factors. Adv Exp Med Biol. 1997;429:69–77. doi: 10.1007/978-1-4757-9551-6_5. [DOI] [PubMed] [Google Scholar]
- 25.Diemel LT, Copelman CA, Cuzner ML. Macrophages in CNS remyelination: friend or foe? Neurochem Res. 1998;23:341–7. doi: 10.1023/a:1022405516630. [DOI] [PubMed] [Google Scholar]
- 26.Grinspan JB, Stern J, Franceschini B, Yasuda T, Pleasure D. Protein growth factors as potential therapies for central nervous system demyelinative disorders. Ann Neurol. 1994;36(Suppl.):S140–2. doi: 10.1002/ana.410360734. [DOI] [PubMed] [Google Scholar]
- 27.Webster HD. Growth factors and myelin regeneration in multiple sclerosis. Mult Scler. 1997;3:113–20. doi: 10.1177/135245859700300210. [DOI] [PubMed] [Google Scholar]
- 28.Furie MB, Randolph GJ. Chemokines and tissue injury. Am J Pathol. 1995;146:1287–301. [PMC free article] [PubMed] [Google Scholar]
- 29.Taub DD, Turcovski Corrales SM, Key ML, Longo DL, Murphy WJ. Chemokines and T lymphocyte activation: I. Beta chemokines costimulate human T lymphocyte activation in vitro. J Immunol. 1996;156:2095–103. [PubMed] [Google Scholar]
- 30.Wong M, Fish EN. RANTES and MIP-1alpha activate stats in T cells. J Biol Chem. 1998;273:309–14. doi: 10.1074/jbc.273.1.309. [DOI] [PubMed] [Google Scholar]
- 31.Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5 (+) and CXCR3 (+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA. 1999;96:6873–8. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorensen TL, Tani M, Jensen J, et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest. 1999;103:807–15. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnes DA, Huston M, Holmes R, Benveniste EN, Yong VW, Scholz P, Perez HD. Induction of RANTES expression by astrocytes and astrocytoma cell lines. J Neuroimmunol. 1996;71:207–14. doi: 10.1016/s0165-5728(96)00154-3. [DOI] [PubMed] [Google Scholar]
- 34.McManus CM, Brosnan CF, Berman JW. Cytokine induction of MIP-1 alpha and MIP-1 beta in human fetal microglia. J Immunol. 1998;160:1449–55. [PubMed] [Google Scholar]


