In clearing specific types of infection, the immune system mounts a characteristic array of immune responses. Viruses are controlled predominantly by natural killer (NK) cells, CD8+ T cells and CD4+ T cells secreting type-1 cytokines (interferon-gamma (IFN-γ)). On the other hand, extracellular pathogens (such as helminths) are predominantly controlled by CD4+ T cells secreting type-2 cytokines (e.g. IL-4 and IL-5), which lead to antibody isotype switching and recruit and activate eosinophils.
These qualitatively different responses have implications for both host and pathogen. The pathogen may manipulate the host immune response away from those which are protective, while inappropriate host responses may cause immunopathological effects [1]. Animal models are invaluable in unravelling the complexities of infectious diseases. However, the animals used for these models are normally housed in ‘specific pathogen-free’ conditions. The same is not true of natural human or animal infections. Previous or coexisting infection is the rule rather than exception, and may indeed explain the diverse outcomes of infection with a given pathogen. For example, a strong immune response to one pathogen can impair responses to other pathogens [2] and co-infections are a major determinant of outcome and life span in HIV+ individuals.
In this issue of Clinical and Experimental Immunology, Hertoghe et al. describe studies of immune parameters in Ugandan patients with HIV and/or active pulmonary tuberculosis (TB). The characteristic features of either disease (e.g. CD4 depletion and apoptosis in HIV+ subjects and monocytosis, granulocytosis and increased PPD-induced apoptosis in TB-infected patients) remained evident in the co-infected individuals, while immune activation (as measured by CD38 display on CD8 cells) was most marked in those with co-infection. Previous attempts to find differences in co-infected patients have not always been successful. For example, soluble serum CD14 is equally raised in the serum of HIV+ patients, with or without TB [3]. Such studies are difficult to do but are much needed if we are to understand the complex interactions of multiple infections.
In many parts of the world HIV and other infections combine to devastating effect. The situation is particularly acute in developing countries, since chronic parasitic, helminthic [4] and bacterial infections put further burdens on the immune system. As a result, HIV may have found its optimum niche in populations where viral replication and disease progression are accelerated by chronic immune activation by co-infecting pathogens. Pulmonary infections are a major cause of death in immunocompromised patients (for a review see [5]), and TB remains the most prevalent cause of death from an infectious agent. TB, caused by Mycobacterium tuberculosis (MTB), is also the most important HIV co-infection, causing 30% of AIDS-associated deaths in 1999 alone [6].
The importance of cell-mediated immunity in acute MTB infection has been repeatedly demonstrated [7]. CD4+ T cells are an absolute requirement for protection. In murine models, the primary mechanism for this is through production of IFN-γ and downstream effector molecules such as reactive nitrogen intermediates [8,9]; without this pathway, fatal tuberculous disease is inevitable. Epidemiologically, the consequences of HIV infection are equally evident in AIDS patients acutely infected with MTB [10]. Upon exposure, HIV-infected and AIDS patients are over 100 times more likely to develop active TB and subsequently have shorter survival times [11, 12]. In latent MTB infections, the chance of a reactivation leading to TB disease is about 10% per year, equal to the lifetime risk for MTB-infected HIV− individuals [13]. It is likely that a large part of this susceptibility is due to insufficient or inadequate responses by CD4+ T cells.
However, recent work suggests that it may not be quite that simple. The activation of MTB-infected macrophages by IFN-γ is a critical part of acquired immunity. Other mechanisms for modulation of macrophage function are dependent on the activation and localization of CD4+ T cells. Although their role in TB is controversial, macrophage activation by CD40–CD40L binding or inhibition by IL-10 and transforming growth factor-beta (TGF-β) could be mediated by CD4+ T cells [14]. Also, MTB-specific CD8+ T cells produce IFN-γ and kill mycobacterially infected cells [15, 16]. Since they do not provide compensatory protection in CD4+ T cell-deficient patients they probably depend on CD4+ T cell help.
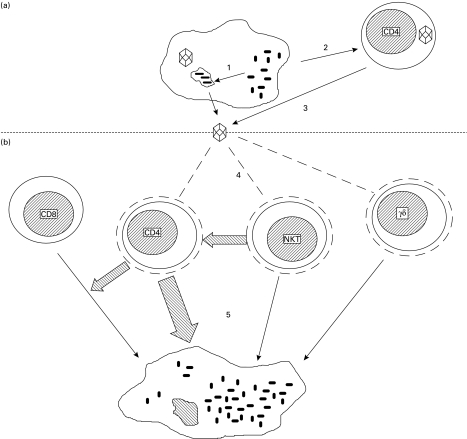
The effects of HIV infection on control of MTB are probably even more complex than this. CD4+ NK cells play a role in the structural organization of granulomas, which are an important part of mycobacterial control. Granulomas become increasingly disorganized in HIV+ individuals, in correlation with their peripheral CD4+ cell count [17, 18]. γδ T cells recognize non-peptidic antigens and are important in immunity against mycobacterial pathogens, including MTB. HIV infection reduces the numbers of circulating γδ T cells and, in a proportion of infected individuals, they also become anergic in their IFN-γ response to MTB [19]. Though the contribution of these and other cell types to immunity is difficult to assess, it is clear there are complex interactions between HIV and host cells that significantly inhibit their ability to control active TB (see Fig. 1).
Fig. 1.
Reciprocal enhancement of replication in HIV and Mycobacterium tuberculosis (MTB) co-infection. (a) MTB infection increases HIV production from co-infected macrophages (1) and activates and causes proliferation of CD4+ T cells (2), which creates more optimal conditions for HIV production from infected lymphocytes (3). (b) HIV reduces circulating CD4+, γδ and natural killer (NK) T cells through a variety of mechanisms (4), which normally kill MTB-infected macrophages (5).
However, the consequences of co-infection do not work only in one direction—the course of HIV infection is also affected by the presence of MTB and the resultant immune response. In vitro and in animal studies, infection with MTB accelerates the replication of HIV. Since the virus preferentially replicates in acutely infected activated CD4+ T cells, infection with mycobacteria, as with other pathogens, will cause replication and activation of these cells and hence increase viral replication [20, 21]. MTB also increases HIV replication in acutely and chronically infected monocytes [22]. The effect of MTB on HIV is therefore due to both immune cell activation and macrophage dysregulation. Though the effect is complex, the consequences are significant. In primate studies co-infection with simian immunodeficiency virus (SIV) and MTB resulted in increased replication of SIV, enhanced decline of peripheral CD4+ cell counts and a faster progression to AIDS [23]. In man, TB, even if successfully treated, increases the risk that HIV-infected individuals will subsequently develop AIDS-defining opportunistic infections [12].
In addition to providing a heavy and diverse burden on the immune system, some infections interact in specific ways to the host's detriment. The identification of target sequences in HIV that interact with human cytomegalovirus provides a specific mechanism for increased HIV replication during co-infection [24]. Those infected with other pathogens, from Neisseria gonhorreae to hepatitis C virus, also suffer from accelerated HIV disease progression [25].
Leishmania donovani antigens increase HIV replication and CD4+ T cell apoptosis [26]. An effective response to anti-parasite treatment correlates with a marked decrease in post-treatment HIV viral load. By contrast, a poor response is associated with an increased viral load; initial HIV viral load also influences the response to anti-leishmanial treatment [27]. In man co-infection with Leishmania spp. and HIV is associated with elevated serum IFN-γ and tumour necrosis factor (TNF), and decreased levels of the immunosuppressive cytokine IL-10 [28]. There is evidence of reciprocal influences on both co-infecting pathogens. For example, live and killed HIV increases the intracellular growth of L. donovani in monocytes [29]. HIV appears to counteract parasite control mechanisms and may be responsible for their reactivation.
The interaction between co-infecting pathogens is particularly evident when they both infect the same antigen-presenting cell [30, 31]. Thus, co-infecting pathogens enhance immune activation and accelerate HIV replication, and further complications occur when the co-infecting pathogen also causes immunosuppression. Onchocerca volvulus infection of HIV-infected patients further decreases proliferation and cytokine production of T cells to polyclonal stimulants [32]. Co-infection with HIV and Treponema pallidum (syphilis also being endemic in West Africa) has also been reported to further reduce CD4+ T cells [33].
There are extra factors, nothing to do with immunology, which have a very real impact on the majority of those infected with HIV. Around a million lives were to lost to HIV and MTB co-infection in 1999 alone [6]. However, less than 1% of the cases of co-infection, let alone deaths, occurred in wealthier countries where drug therapy has greatly reduced morbidity and mortality [34]. In the developing world gross inequalities in wealth lead to uneven healthcare provision. As a result, advances in treatment of TB and AIDS have largely not been implemented. Where they have, the situation has improved; for instance, several African countries have prevented the emergence of multi-drug resistant MTB through directly observed therapy schemes. It is clear that co-infections are the rule rather than the exception—our research and treatment strategies must take this into account if we are to make progress in tackling infectious disease in man.
REFERENCES
- 1.Selin LK, Varga SM, Wong IC, Welsh RM. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med. 1998;188:1705–15. doi: 10.1084/jem.188.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Openshaw PJM. When we sneeze, does the immune system catch a cold? Br Med J. 1991;303:935–6. doi: 10.1136/bmj.303.6808.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawn SD, Labeta MO, Arias M, Acheampong JW, Griffin GE. Elevated serum concentrations of soluble CD14 in HIV− and HIV+ patients with tuberculosis in Africa: prolonged elevation during anti-tuberculosis treatment. Clin Exp Immunol. 2000;120:483–7. doi: 10.1046/j.1365-2249.2000.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentwich Z, Kalinkovich A, Weisman Z, Borkow G, Beyers N, Beyers AD. Can eradication of helminthic infections change the face of AIDS and tuberculosis? Immunol Today. 1999;20:485–7. doi: 10.1016/s0167-5699(99)01499-1. [DOI] [PubMed] [Google Scholar]
- 5.Baughman RP. The lung in the immunocompromised patient. Infectious complications Part 1. Respiration. 1999;66:95–109. doi: 10.1159/000029349. [DOI] [PubMed] [Google Scholar]
- 6.Zumla A, Malon P, Henderson J, Grange JM. Impact of HIV infection on tuberculosis. Postgrad Med J. 2000;76:259–68. doi: 10.1136/pmj.76.895.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahn J, Kaufmann SHE. Immune mechanisms of protection. In: Bloom BR, editor. Tuberculosis: pathogenesis, protection and control. Washington DC: American Society for Microbiology; 1994. pp. 389–415. [Google Scholar]
- 8.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon gamma genes. Science. 1993;259:1739–42. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 10.Ottenhoff TH, Kumararatne D, Casanova JL. Novel human immunodeficiencies reveal the essential role of type-1 cytokines in immunity to intracellular bacteria. Immunol Today. 1998;19:491–4. doi: 10.1016/s0167-5699(98)01321-8. [DOI] [PubMed] [Google Scholar]
- 11.Villarino ME, Dooley SW, Geiter LJ, Castro KG, Snider De., Jr Management of persons exposed to multidrug resistant tuberculosis. Morbidity Mortality Weekly Report. 1992;41:61. [PubMed] [Google Scholar]
- 12.Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–35. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 13.Miller R. HIV-associated respiratory diseases. Lancet. 1996;348:307–12. doi: 10.1016/s0140-6736(95)11037-2. [DOI] [PubMed] [Google Scholar]
- 14.Scanga CA, Mohan VP, Yu K, et al. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J Exp Med. 2000;192:347–58. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller I, Cobbold SP, Waldmann H, Kaufmann SH. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987;55:2037–41. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lalvani A, Brookes R, Wilkinson RJ, et al. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1998;95:270–5. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Perri G, Cazzadori A, Vento S, et al. Comparative histopathological study of pulmonary tuberculosis in human immunodeficiency virus-infected and non-infected patients. Tuber Lung Dis. 1996;77:244–9. doi: 10.1016/s0962-8479(96)90008-8. [DOI] [PubMed] [Google Scholar]
- 18.Saunders BM, Frank AA, Cooper AM, Orme IM. Role of gamma delta T cells in immunopathology of pulmonary Mycobacterium avium infection in mice. Infect Immun. 1998;66:5508–14. doi: 10.1128/iai.66.11.5508-5514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boullier S, Poquet Y, Debord T, Foumie JJ, Gougeon ML. Regulation by cytokines (IL-12, IL-15, IL-4 and IL-10) of the Vgamma9Vdelta2 T cell response to mycobacterial phosphoantigens in responder and anergic HIV-infected persons. Eur J Immunol. 1999;29:90–99. doi: 10.1002/(SICI)1521-4141(199901)29:01<90::AID-IMMU90>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Heng MC, Heng SY, Allen SG. Co-infection and synergy of human immunodeficiency virus-1 and herpes simplex virus-1. Lancet. 1994;343:255–8. doi: 10.1016/s0140-6736(94)91110-x. [DOI] [PubMed] [Google Scholar]
- 21.Claydon EJ, Bennett J, Gor D, Forster SM. Transient elevation of serum HIV antigen levels associated with intercurrent infection [letter] AIDS. 1991;5:113–4. doi: 10.1097/00002030-199101000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Nakata K, Weiden M, Rom WN. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication by transcriptional activation at the long terminal repeat. J Clin Invest. 1995;95:2324–31. doi: 10.1172/JCI117924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou D, Shen Y, Chalifoux L, et al. Mycobacterium bovis bacille Calmette–Guerin enhances pathogenicity of simian immunodeficiency virus infection and accelerates progression to AIDS in macaques: a role of persistent T cell activation in AIDS pathogenesis. J Immunol. 1999;162:2204–16. [PubMed] [Google Scholar]
- 24.Yurochko AD, Huong SM, Huang ES. Identification of human cytomegalovirus target sequences in the human immunodeficiency virus long terminal repeat. Potential role of 1E2-86 binding to sequences between -120 and -20 in promoter transactivation. J Hum Virol. 1999;2:81–90. [PubMed] [Google Scholar]
- 25.Piroth L, Duong M, Quantin C, et al. Does hepatitis C virus co-infection accelerate clinical and immunological evolution of HIV-infected patients? AIDS. 1998;12:381–8. doi: 10.1097/00002030-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Wolday D, Akuffo R, Demissie A, Britton S. Role of Leishmania donovani and its lipophosphoglycan in CD4+ T-cell activation-induced human immunodeficiency virus replication. Infect Immun. 1999;67:5258–64. doi: 10.1128/iai.67.10.5258-5264.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berhe N, Wolday D, Hailu A, et al. HIV viral load and response to antileishmanial chemotherapy in co-infected patients. AIDS. 1999;13:1921–5. doi: 10.1097/00002030-199910010-00015. [DOI] [PubMed] [Google Scholar]
- 28.Yurochko AD, Huong SM, Huang ES. Identification of human cytomegalovirus target sequences in the human immunodeficiency virus long terminal repeat. Potential role of 1E2-86 binding to sequences between -120 and -20 in promoter transactivation. J Hum Virol. 1999;2:81–90. [PubMed] [Google Scholar]
- 29.Wolday D, Akuffo H, Fessahaye G, Valantine A, Britton S. Live and killed human immunodeficiency virus type-1 increases the intracellular growth of Leishmania donovani in monocyte-derived cells. Scand J Infect Dis. 1998;30:29–34. doi: 10.1080/003655498750002268. [DOI] [PubMed] [Google Scholar]
- 30.Wolday D, Berhe N, Akuffo H, Britton S. Leishmania–HIV interaction: immunopathogenic mechanisms. Parasitol Today. 1999;15:182–7. doi: 10.1016/s0169-4758(99)01431-3. [DOI] [PubMed] [Google Scholar]
- 31.Wahl SM, Greenwell WT, Peng G, Hale DH, Orenstein JM. Co-infection with opportunistic pathogens promotes human immunodeficiency virus type 1 infection in macrophages. J Infect Dis. 1999;179(Suppl. 3):S457–S460. doi: 10.1086/314814. [DOI] [PubMed] [Google Scholar]
- 32.Sentongo E, Rubaale T, Buttner DW, Brattig NW. T cell responses in coinfection with Onchocerca volvulus and the human immunodeficiency virus type 1. Parasite Immunol. 1998;20:431–9. doi: 10.1046/j.1365-3024.1998.00170.x. [DOI] [PubMed] [Google Scholar]
- 33.N'gom PT, Jaffar S, Ricard D, et al. Immune stimulation by syphilis and malaria in HIV-2-infected and uninfected villagers in West Africa. Br J Biomed Sci. 1997;54:251–5. [PubMed] [Google Scholar]
- 34.Ashley EA, Johnson MA, Lipman MC. Human immunodeficiency virus and respiratory infection. Curr Opin Pulm Med. 2000;6:240–5. doi: 10.1097/00063198-200005000-00013. [DOI] [PubMed] [Google Scholar]