Abstract
The mechanism by which Burkholderia pseudomallei survives in macrophages is not clearly understood. In this study, we demonstrated that the mouse macrophage cell line (RAW 264.7) treated with lipopolysaccharide (LPS) from B. pseudomallei (BP-LPS) produced significantly less NO and TNF-α compared with those stimulated with the LPS from Escherichia coli and Salmonella typhi. The time required for the BP-LPS to trigger substantial NO and TNF-α release was at least 30 min, compared with <5 min for the E. coli-LPS. A time course study of inducible nitric oxide synthase (iNOS) protein expression also indicated that the time required for macrophages stimulated with the BP-LPS to up-regulate iNOS was longer. The longer time lag for the BP-LPS to activate macrophages was probably due to the delay in up-regulation of iNOS and TNF-α mRNA transcription. These results indirectly suggest that the delay of the mediators' production may be due to a reduced rate of signal transduction initiated by the interaction of BP-LPS with the macrophage cell surface. The use of MoAb to phosphorylated p38 in a Western blot analysis provided data compatible with the notion that the maximum level of phosphorylated p38 from the cells activated with BP-LPS was attained at a slower rate. These results suggest that the unique structure of BP-LPS exhibits a property which may interfere with macrophage cell activation.
Keywords: nitric oxide, inducible nitric oxide synthase, tumour necrosis factor-alpha, melioidosis, lipopolysaccharide, Burkholderia pseudomallei
INTRODUCTION
Melioidosis, a bacterial disease caused by Burkholderia pseudomallei, is endemic in tropical areas such as south-east Asia, northern Australia and other temperate regions that border the equator [1–4]. Acquisition of melioidosis occurs via inoculation of the damaged tissue surface with contaminated soil or water; or by inhalation and aspiration of contaminated dust particles [5]. Infection by this Gram-negative bacterium may cause acute septicaemic melioidosis which affects various organs throughout the body, particularly the lungs, liver, spleen and lymph nodes [4]. In acute septicaemia, there are fever, chill, muscle pain and other signs and symptoms resulting from localized abscess. Systemic infection is associated with a high mortality rate, slow response to anti-microbial therapy and high rate of relapse despite prolonged treatment [6]. Subclinical or asymptomatic infection is the most common form of melioidosis [3]. In some cases the bacteria remain latent and clinical disease can relapse after a long period of latency [7].
The pathogenesis of B. pseudomallei has also been extensively studied in the murine system. Murine macrophage responses to B. pseudomallei may vary from one strain to another, i.e. BALB/c mice are more susceptible to B. pseudomallei than C57Bl/6 mice [8]. The levels of mRNA for several cytokines such as interferon-gamma (IFN-γ) were higher in BALB/c mice infected with B. pseudomallei than in C57Bl/6 [8]. Moreover, several groups of investigators [9,10] have demonstrated that B. pseudomallei can survive and multiply in mouse macrophages. Bactericidal activity in mouse macrophages correlated with the production of NO [10]. Inhibition of NO production by a NO inhibitor such as NG-monomethyl l-arginine (L-NMMA) suppressed B. pseudomallei multiplication in mouse macrophage cell line. Among the many virulence factors of Gram-negative bacteria, lipopolysaccharide (LPS) is a major contributing factor in systemic sepsis and tissue injury [11]. It is well documented that the LPS activates macrophages and induces a number of molecules including reactive nitrogen intermediates and cytokines (e.g. TNF-α, IL-1 and IL-6) both in vivo and in vitro [12]. The macrophage response to LPS is initiated by the binding of LPS and lipopolysaccharide binding protein (LBP) complex with CD14. Within 1 min of exposure, the LPS is able to bind to the CD14 on the cell surface [13]. The time required to trigger the mediator production (such as TNF-α) from the monocytes activated by LPS is only 5–15 min [13].
LPS isolated from B. pseudomallei (BP-LPS) has been reported to exhibit weaker macrophage activation activity than enterobacterial LPS by at least one order of magnitude [14]. On the other hand, the BP-LPS appears to have stronger mitogenic activity for murine splenocytes than the enterobacterial LPS. The BP-LPS also has an unusual acid stable structure in the inner core region attached to the lipid A moiety [15]. Whether this unique structure can influence its biological activity or how this unique LPS which has a weaker macrophage activation potential plays a role in pathogenesis of B. pseudomallei remains to be investigated. In the present study, we investigated the kinetics of NO and TNF-α release and the kinetics of inducible nitric oxide synthase (iNOS) and TNF-α gene expression from mouse macrophage cells (RAW 264.7) activated with BP-LPS.
MATERIALS AND METHODS
Cell line and culture condition
Mouse macrophage cell line (RAW 264.7) was obtained from American Type Culture Collection (ATCC, Rockville, MD). If not indicated otherwise, the cells were cultured in Dulbecco's modified Eagles' medium (DMEM; Gibco Labs, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT) and grown at 37°C under a 5% CO2 atmosphere.
Bacteria isolation
Burkholderia pseudomallei 844 (arabinose-negative strain) was isolated from patients admitted to Srinakarind Hospital in the melioidosis-endemic Khon Kaen province of Thailand. The bacterium was originally identified as B. pseudomallei based on its biochemical characteristics, colonial morphology on selective media, antibiotic sensitivity profiles and reaction with polyclonal antibody [16]. The Escherichia coli and Salmonella typhi used for comparison throughout these experiments were originally obtained from Ramathibodi Hospital (Mahidol University, Bangkok, Thailand) and kept as stock culture in our laboratory.
Preparation of LPS
LPS was extracted from B. pseudomallei, E. coli and S. typhi isolates by the modified phenol-chloroform-petroleum ether method [15] and characterized by SDS–PAGE and immunoblotting as previously reported [17]. The LPS from E. coli strain 0111:B4 (Sigma, St Louis, MO) was also used as reference. The carbohydrate content of these LPSs was determined by the orcinol-sulphuric acid method using glucose as standard [18].
Limulus amoebocyte lysate assay
The biological activity of LPS was determined by Limulus amoebocyte lysate (LAL) assay. The tests were performed essentially as recommended by the manufacturer (BioWhitaker, Walkersville, MD) with E. coli-LPS as standard.
Treatment of mouse macrophage cell line (RAW 264.7) with LPS
Mouse macrophage cells (1 × 106) were exposed to various concentrations of BP-LPS and E. coli-LPS. Eighteen hours after exposure, the supernatant was analysed for NO and TNF-α release. To determine for iNOS protein, the cells were lysed with reducing buffer (62·5 mm Tris pH 6·8, 6 m urea, 10% glycerol, 2% SDS, 0·003% bromphenol blue and 5% 2-mercaptoethanol) followed by sonication on ice for 20 s. Twenty microlitres of samples were subjected to SDS–PAGE and transferred to PVDF membrane (BioRad, Hercules, CA). The iNOS protein was detected by immunoblotting using MoAb specific to iNOS (Santa Cruz, Santa Cruz, CA).
NO assay
The production of NO was determined by measuring the quantity of nitrite in the supernatant by the Griess reaction [19].
TNF-α assay
TNF-α activity was measured by a cytotoxic assay against L-929 [20]. Treated cells were stained with crystal violet after 18 h. Change in absorbance at 540 nm was measured and converted to unit per millilitre of TNF-α based on a standard curve using murine TNF-α (Genzyme, Cambridge, MA) as standard.
Reverse transcriptase-polymerase chain reaction
Mouse macrophage cells (3 × 106) were stimulated with BP-LPS (100 ng/ml) and E. coli-LPS (10 ng/ml) for 15, 30, 60 and 120 min at 37°C before replacing with media containing only 10% FBS. After 9 h, the cells were extracted with trizol reagent (Gibco Labs) for total RNA isolation. The extracted RNA was subsequently treated with DNase (Promega, Madison, WI) according to the manufacturer's instructions before use for cDNA synthesis by AMV reverse transcriptase (Promega) [21].
The PCR reaction was conducted by using cDNA as template for iNOS and TNF-α amplification by a GeneAmp PCR System 2400 (Perkin Elmer, Norwalk, CT). The primers for iNOS were: sense 5′-CCG AAG TTT CTT GTG GCA GCA GCG-3′, antisense 5′-GAG CCT CGT GGC TTT GGG CTC CTC-3′; and for TNF-α were: sense 5′-AGC CCA CGT CGT AGC AAA CCA CCA A-3′, antisense 5′-ACA CCC ATT CCC TTC ACA GAG CAA T-3′. The amplified products were electrophoresed on 1·8% agarose gel before being transferred to Hybond-N+ membrane (Amersham, Aylesbury, UK). The membranes were prehybridized in buffer containing 1% bovine serum albumin (BSA), 7% SDS, 1 mm EDTA, 0·5 m phosphate buffer at 60°C for 2 h prior to hybridizing at 60°C overnight with radiolabelling 32P-ATP oligonucleotide probes of iNOS (5′-ACG TTC AGG ACA TCC TGA AAA AGC AGC TGG-3′) or TNF-α (5′-CTG GAA GAC TCC TCC CAG GTA TAT GGG-3′). Thereafter, the membranes were washed and subjected to autoradiography as described [21].
Western blot analysis for phosphorylated p38
Mouse macrophage cells (1 × 107) were activated with BP-LPS (100 ng/ml) or E. coli-LPS (10 ng/ml) for 5, 15, 30 and 60 min at 37°C. After stimulation, the cells were washed twice with ice-cold PBS containing 1 mm Na3VO4. The cells were lysed with 400 μl of ice-cold lysis buffer (20 mm Tris–HCl pH 7·5, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 2·5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 μg/ml leupeptin) [22]. The lysates (35 μg) were subjected to electrophoresis in 10% SDS–PAGE before being transferred to PVDF membrane. The membrane was blocked in 5% skim milk for 1 h and followed by reacting with MoAb specific to phosphorylated p38 (Santa Cruz) at 4°C overnight. The membrane was then reacted with horseradish peroxidase-conjugated rabbit anti-mouse IgG (Dako, Glostrup, Denmark) for 1 h. The reaction was detected by enhanced chemiluminescence as recommended by the manufacturer (Pierce, Rockford, IL).
RESULTS
Characterization of purified LPS preparations
The LPS preparations purified as described and used throughout this study were extracted from pathogenic B. pseudomallei, E. coli, and S. typhi. The purity of the extracted LPSs was determined by SDS–PAGE. The result on silver staining showed a ladder pattern typical of Gram-negative bacterial LPS. There was no detectable protein band contamination, judging from coomassie blue staining (data not shown). The endotoxic activity, determined by LAL assay, of BP-LPS and E. coli-LPS was 4 × 104 and 1·1 × 105 EU/ml, respectively. The data obtained with these E. coli-LPS preparations were identical to those of the commercial E. coli-LPS used for comparison.
Activation of mouse macrophage cell line (RAW 264.7) with BP-LPS
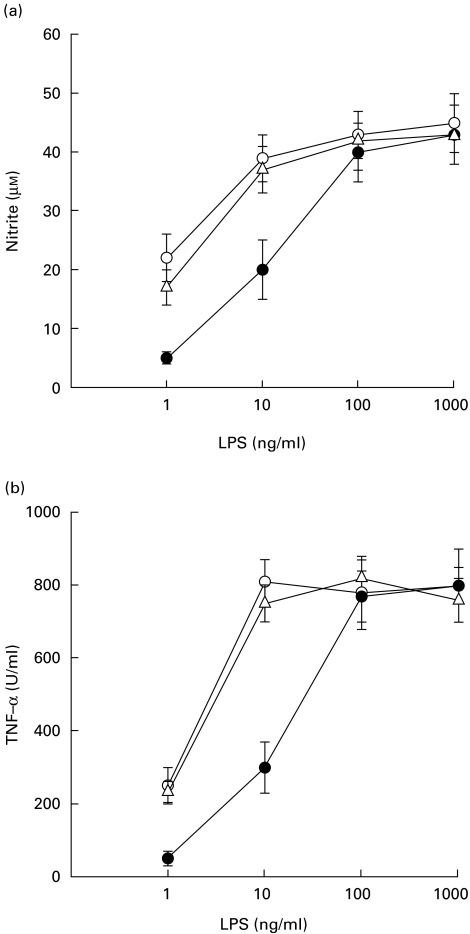
To determine if the LPS isolated from B. pseudomallei could induce NO and TNF-α release, the macrophage cells were first exposed to various concentrations of BP-LPS at 37°C for 18 h and the supernatant was then analysed for NO and TNF-α. When these cells were stimulated with the BP-LPS, the production of these mediators was detectable from a dose as low as 1 ng/ml, reaching a plateau level at 100 ng/ml, while those of the cells stimulated with the E. coli-LPS or S. typhi-LPS reached the plateau level at 10 ng/ml (Fig. 1). Judging from these results, it appears that the macrophage-activating activity of BP-LPS was one order of magnitude weaker than those of E. coli-LPS or S. typhi-LPS. Based upon these findings, subsequent experiments were carried out using 100 ng/ml of BP-LPS and 10 ng/ml of E. coli-LPS.
Fig. 1.
NO and TNF-α release from mouse macrophage cells (RAW 264.7) activated by lipopolysaccharide (LPS). The macrophage cells were treated with various concentrations of LPS from Burkholderia pseudomallei (BP-LPS) (•), Escherichia coli-LPS (○) or S. typhi-LPS (Δ). After 18 h of activation, the supernatant was analysed for NO (a) and TNF-α release (b). Each point represents the mean and s.d. of five separate experiments with duplicated samples.
Time required for NO and TNF-α release by mouse macrophage cell line (RAW 264.7) activated with BP-LPS
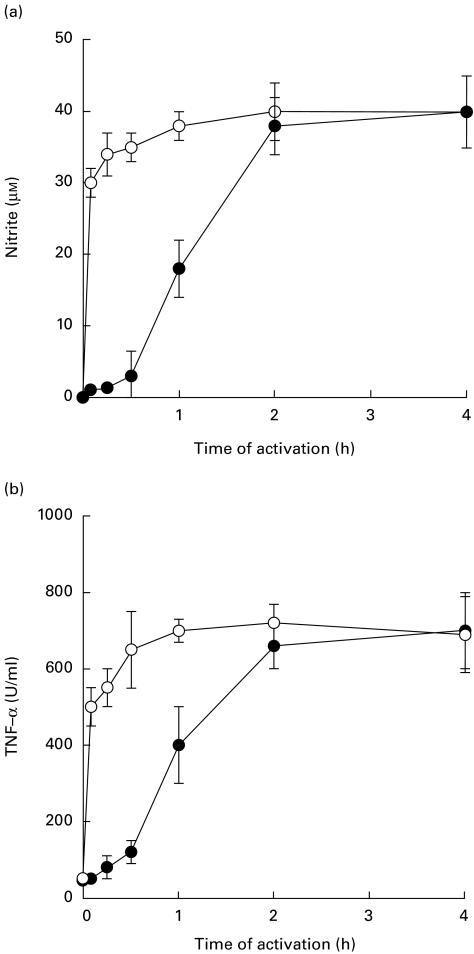
Mouse macrophages were stimulated with either BP-LPS (100 ng/ml) or E. coli-LPS (10 ng/ml) for 5, 15, 30 min, 1, 2 and 4 h. The supernatants were at times indicated, analysed for NO and TNF-α. The results showed that the BP-LPS stimulated NO or TNF-α release at a considerably slower rate (Fig. 2). The time required to activate NO or TNF-α release was between 30 min and 60 min before reaching a plateau after 2 h. Unlike the BP-LPS, E. coli-LPS exhibited a faster kinetic rate. The time required to stimulate NO or TNF-α release was detected within 5 min and reached a plateau level within 30–60 min after activation. Although the BP-LPS concentration used was 10 times higher than that of the E. coli-LPS, the maximum concentrations of NO and TNF-α release from macrophages activated by these two LPSs were not different from one another, as shown in Fig. 1.
Fig. 2.
Kinetics of NO and TNF-α release from mouse macrophage cells (RAW 264.7) activated by lipopolysaccharide (LPS). Macrophage cells were activated with LPS from Burkholderia pseudomallei (BP-LPS; 100 ng/ml) (•) or Escherichia coli-LPS (10 ng/ml) (○) for 5, 15, 30 min, 1, 2 and 4 h. The cells were washed with PBS three times to remove excess LPS and then incubated further in medium containing 10% fetal bovine serum. After 18 h of activation, the supernatant was analysed for NO (a) or TNF-α release (b). Each point represents the mean and s.d. of five separate experiments with duplicated samples.
Kinetics of iNOS protein production from mouse macrophage cell line (RAW 264.7) activated by BP-LPS
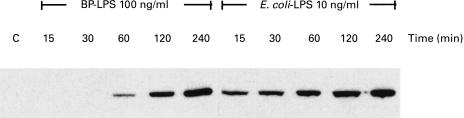
The slower rate of NO released from the BP-LPS-activated macrophages indirectly implied that the BP-LPS may up-regulate the iNOS expression at a slower rate. The results in Fig. 3 show that the presence of iNOS protein was observed from the cells activated with BP-LPS only after 30 min of LPS exposure. Under similar conditions, the presence of iNOS protein in the cells treated with E. coli-LPS was detected within 15 min of activation.
Fig. 3.
Kinetics of inducible nitric oxide synthase (iNOS) protein expression from mouse macrophage cells (RAW 264.7) activated by lipopolysaccharide (LPS). The macrophage cells were activated with LPS from Burkholderia pseudomallei (BP-LPS; 100 ng/ml) or Escherichia coli-LPS (10 ng/ml) for 15, 30, 60 and 120 min. After 18 h of incubation, the samples were lysed in lysis buffer and the lysate was subjected to immunoblotting analysis with MoAb to iNOS. Data are representative of three independent experiments with similar results.
Kinetics of iNOS and TNF-α mRNA expression from mouse macrophage cell line (RAW 264.7) activated by BP-LPS
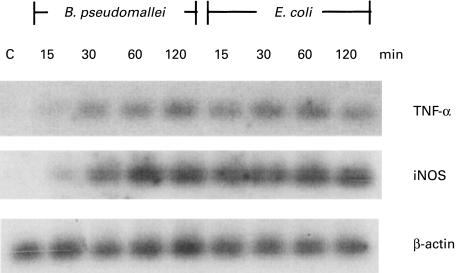
The delay of mediators released from BP-LPS-activated macrophages indirectly implied that the BP-LPS may require a longer time to up-regulate the mRNA of iNOS and TNF-α. The results in Fig. 4 show that the cells activated with the BP-LPS (100 ng/ml) slowly up-regulated the mRNA of both iNOS and TNF-α, while those treated with the E. coli-LPS expressed almost a maximum level of iNOS and TNF-α mRNA within 15 min. The level of β-actin, which served as an internal control, was not influenced by LPS.
Fig. 4.
Kinetics of inducible nitric oxide synthase (iNOS) and TNF-α mRNA expression from mouse macrophage cells (RAW 264.7) activated by lipopolysaccharide (LPS). The macrophage cells were activated with LPS from Burkholderia pseudomallei (BP-LPS; 100 ng/ml) or Escherichia coli-LPS (10 ng/ml) for 15, 30, 60 and 120 min. After 9 h of incubation, the synthesized cDNA from macrophage cell RNA was subjected to polymerase chain reaction following by hybridization with appropriate radiolabelled probes. The β-actin served as an internal control. Data are representative of three independent experiments with similar results.
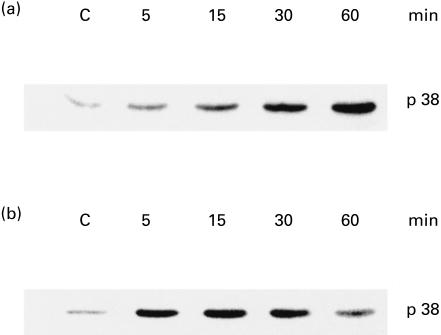
Time course studies of p38 phosphorylation in BP-LPS-activated macrophages
The kinetic difference in the production of NO and TNF‐α by the macrophages activated with BP-LPS and E. coli-LPS may have been caused by interference at a stage of signal transduction. This possibility was investigated by comparing the levels of p38 phosphorylation at different time points. To achieve this, the macrophage cells were activated with the BP-LPS (100 ng/ml) or E. coli-LPS (10 ng/ml) for 5, 15, 30 and 60 min. Immediately, the cells were lysed in lysis buffer, followed by immunoblotting using MoAb to p38. The results showed that the p38 was gradually phosphorylated and reached a maximum after 60 min following activation by BP-LPS (Fig. 5a). On the other hand, the phosphorylation of p38 in the cells activated with E. coli-LPS reached the maximum only after 5–15 min of activation (Fig. 5b). In the E. coli system, the level of phosphorylated p38 began to decline after 30 min of activation. These results suggest that the activation of cells by BP-LPS had a longer time delay of signal transduction compared with cells activated with E. coli-LPS.
Fig. 5.
Phosphorylation of p38 from mouse macrophage cell (RAW 264.7) activated by lipopolysaccharide (LPS). The macrophage cells were treated with LPS from Burkholderia pseudomallei (BP-LPS; 100 ng/ml) (a) or Escherichia coli-LPS (10 ng/ml) (b) for 5, 15, 30 and 60 min. Phosphorylation of p38 was analysed by immunoblotting using MoAb to phosphorylated p38. Data are representative of three independent experiments with similar results.
DISCUSSION
LPS is a component of the outer cell membrane of Gram-negative bacteria that is an important factor mediating the production and release of various mediators (such as NO) and cytokines (such as TNF-α) from macrophages through CD14 [12]. In human mononuclear cells, neutralizing antibody to CD14 was able to inhibit the binding of fluorescence isothiocyanate-conjugated BP-LPS and E. coli-LPS, indicating that BP-LPS interact with CD14 on the macrophage surface (data not shown). Although the BP-LPS may share a common receptor with E. coli-LPS, the data of this study show that the former exhibited macrophage-activating activity one order of magnitude weaker than that of the E. coli and S. typhi. This conclusion confirmed and extended the data previously reported by Matsuura et al. [14]. In subsequent experiments, the BP-LPS was therefore used at a concentration 10 times higher than that of the E. coli-LPS (100 ng/ml and 10 ng/ml, respectively). Under these conditions, we demonstrated that the kinetics of NO and TNF-α release from macrophages activated by BP-LPS was slower than that of the macrophages activated by E. coli-LPS (Fig. 2) or S. typhi-LPS (data not shown). The delay of NO release correlated with the rate of iNOS protein expression (Fig. 3). In addition to this, prolongation at the transcriptional level of both iNOS and TNF-α mRNA was also detected (Fig. 4). These results indirectly suggest that compared with the E. coli-LPS, signal transduction initiated by the interaction of BP-LPS to the cell surface component(s) had been delayed.
Mammalian cells have at least three major MAP kinase pathways that act as relays between extracellular signals and transcription factor [23]. Among them, p38 protein is known to play an important role in signal transduction by LPS, such as demonstrated in TNF-α expression in LPS-stimulated macrophages [24,25]. Phosphorylation of p38 occurred rapidly after the cells were activated with E. coli-LPS [26]. The level of phosphorylation declined after 30–60 min of activation [27]. However, the delay of phosphorylated p38 was observed in the cells activated by BP-LPS (Fig. 5). This result is consistent with the delay of mRNA expression (Fig. 4) and the release of both NO and TNF-α (Fig. 2). Such a delay of signalling transduction may have contributed to the longer time required for the mediators' release. The delay of signal transduction in macrophages activated with BP-LPS may be related to the unique structure of LPS of B. pseudomallei. It has been reported that the BP-LPS exhibits an unusual chemical structure in the acid stable inner core region attached to the lipid A moiety [15]. The difference of kinetics of mediator release and the delay of signal transduction level may be associated with this uncommon structure of the BP-LPS. This unique structure may affect the binding affinity of LPS with the cell receptors, which could result in its weaker macrophage-activating activity.
Burkholderia pseudomallei has been demonstrated to survive and multiply in both phagocytic and non-phagocytic cells [9,28,29]. However, this Gram-negative bacterium was previously shown to be highly susceptible to reactive nitrogen intermediates produced by macrophage cells such as NO [10]. In the present study, we have demonstrated that the BP-LPS not only stimulates NO and TNF-α production inefficiently, but also activates their production and release with a slower kinetic rate compared with the E. coli-LPS. The slow release of mediators could have been caused by the delay of signal transduction, which may in turn influence the rate of mediator synthesis. Possession of weaker and slower macrophage activation activities may facilitate the survival of B. pseudomallei in the macrophages, thus helping it to evade the host defensive system and allowing it to survive for a long time after initial exposure.
Acknowledgments
This work was supported by research grants from Thailand Research Fund and Chulabhorn Research Institute (Thailand).
REFERENCES
- 1.Chaowagul W, White NJ, Dance DAB, Wattanagoon Y, Naigowit P, Davis TME, Looareesuwan S, Pitawatchara N. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J Infect Dis. 1989;159:890–9. doi: 10.1093/infdis/159.5.890. [DOI] [PubMed] [Google Scholar]
- 2.Howe C, Sampath A, Spotnitz M. The pseudomallei group: a review. J Infect Dis. 1971;124:598–604. doi: 10.1093/infdis/124.6.598. [DOI] [PubMed] [Google Scholar]
- 3.Leelarasamee S, Bovornkitti S. Melioidosis: review and update. Rev Infect Dis. 1989;11:413–25. doi: 10.1093/clinids/11.3.413. [DOI] [PubMed] [Google Scholar]
- 4.Yabuuchi E, Arakawa M. Burkholderia pseudomallei and melioidosis: be aware in temperate area. Microbiol Immunol. 1993;37:823–36. doi: 10.1111/j.1348-0421.1993.tb01712.x. [DOI] [PubMed] [Google Scholar]
- 5.Dance DAB, Davis TME, Wattanagoon Y. Acute suppurative parotitis caused by Pseudomonas pseudomallei in children. J Infect Dis. 1989;159:654–60. doi: 10.1093/infdis/159.4.654. [DOI] [PubMed] [Google Scholar]
- 6.White NJ, Dance DAB, Chaowagul W, Wattanagoon Y, Wuthiekanun V, Pitakwatchara N. Halving the mortality of severe melioidosis by ceftazidime. Lancet. 1989;ii:697–700. doi: 10.1016/s0140-6736(89)90768-x. [DOI] [PubMed] [Google Scholar]
- 7.Mays EE, Ricketts EA. Melioidosis: recrudescence associated with bronchogenic carcinoma twenty-six years following initial geographic exposure. Chest. 1975;68:261–3. doi: 10.1378/chest.68.2.261. [DOI] [PubMed] [Google Scholar]
- 8.Ulett GC, Ketheensan N, Hirst RG. Cytokine gene expression in innately susceptible BALB/C mice and relatively resistant C57BL/6 mice during infection with virulent Burkholderia pseudomallei. Infect Immun. 2000;64:2034–42. doi: 10.1128/iai.68.4.2034-2042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones AL, Beveridge TJ, Woods DE. Intracellular survival of Burkholderia pseudomallei and other Burkholderia species on eukaryotic cells in tissue culture. Microbios. 1998;96:71–93. [PubMed] [Google Scholar]
- 10.Miyagi K, Kawakami K, Saito A. Role of reactive nitrogen and oxygen intermediates in gamma interferon-stimulated murine macrophage bactericidal activity against Burkholderia pseudomallei. Infect Immun. 1997;65:4108–13. doi: 10.1128/iai.65.10.4108-4113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandtzaeg P, Kierult P, Gaustad P, Skulberg A, Brunn JN, Halvorsen S, Sorensen E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler-Heitbrock HW, Ulevitch RJ. CD14 cell surface receptor and differentiation marker. Immunol Today. 1993;14:121–5. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]
- 13.Gallay P, Jongeneel CV, Barras C, Burmier M, Baumgartner J, Glauser MP, Heumann D. Short time exposure to lipopolysaccharide is sufficient to activate human monocytes. J Immunol. 1993;150:5086–93. [PubMed] [Google Scholar]
- 14.Matsuura M, Kawahara K, Ezaki T, Nakano M. Biological activities of lipopolysaccharide of Burkholderia (Pseudomonas) pseudomallei. FEMS Microbiol Lett. 1996;137:79–83. doi: 10.1111/j.1574-6968.1996.tb08086.x. [DOI] [PubMed] [Google Scholar]
- 15.Kawahara K, Dejsirilert S, Danbara H, Ezaki T. Extraction and characterization of lipopolysaccharide from Pseudomonas pseudomallei. FEMS Microbiol Lett. 1992;96:129–34. doi: 10.1016/0378-1097(92)90392-2. [DOI] [PubMed] [Google Scholar]
- 16.Wuthiekanun V, Smith MD, Dance DAB, Walsh AL, Pitt TL, White NJ. Biochemical characteristics of clinical and environmental isolates of Burkholderia pseudomallei. J Med Microbiol. 1996;45:408–12. doi: 10.1099/00222615-45-6-408. [DOI] [PubMed] [Google Scholar]
- 17.Anuntagool N, Intachote P, Wuthiekanun V, White NJ, Sirisinha S. Lipopolysaccharide from nonvirulent Ara+ Burkholderia pseudomallei isolates is immunologically indistinguishable from lipopolysaccharide from virulent Ara− clinical isolates. Clin Diagn Lab Immunol. 1998;5:225–9. doi: 10.1128/cdli.5.2.225-229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White CA, Kennedy JF. Oligosaccharides. In: Chaplin MF, Kennedy JF, editors. Carbohydrate analysis: a practical approach. Oxford: IRL Press; 1986. pp. 37–54. [Google Scholar]
- 19.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 20.Ruff MR, Gifford GE. Purification and physiochemical characterization of rabbit tumor necrosis factor. J Immunol. 1980;125:1671–7. [PubMed] [Google Scholar]
- 21.Ubol S, Sukwattanapan C, Utaisincharoen P. Rabies virus replication induces Bax-related, caspase dependent apoptosis in mouse neuroblastoma cells. Virus Res. 1998;56:207–15. doi: 10.1016/s0168-1702(98)00078-1. [DOI] [PubMed] [Google Scholar]
- 22.Mohr S, McCormick TS, Lapetina EG. Macrophages resistant to endogenously generated nitric oxide-mediated apoptosis are hypersensitive to exogenously added nitric oxide donors: dichotomous apoptotic response independent of caspase 3 and reversal by the mitogen-activated protein kinase (MEK) inhibitor PD 098059. Proc Natl Acad Sci USA. 1998;95:5045–50. doi: 10.1073/pnas.95.9.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Triesman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–15. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 24.Han J, Lee JD, Bibbs L, Ulevitch RJA. MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–11. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 25.Han J, Brown T, Beutler B. Endotoxin-responsive sequence control cachectin/ tumor necrosis factor biosynthesis at the translation level. J Exp Med. 1990;171:465–75. doi: 10.1084/jem.171.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J, Lee JD, Tobias PS, Ulevitch RJ. Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem. 1993;268:25009–14. [PubMed] [Google Scholar]
- 27.Weinstein SL, Gold MR, DeFranco AL. Bacterial lipopolysaccharide stimulates protein tyrosine phosphorylation in macrophage. Proc Natl Acad Sci USA. 1991;88:4148–52. doi: 10.1073/pnas.88.10.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukuhara H, Ishimine T, Futeuma M, Saito A. Efficacy of antibiotics against extracellular and intracellular Burkholderia pseudomallei and their therapeutic effects on experimental pneumonia in mice. Jpn J Trop Med Hyg. 1995;23:1–7. [Google Scholar]
- 29.Harley VS, Dance DAB, Drasar BS, Tovey G. Effects of Burkholderia pseudomallei and other Burkholderia species on eukaryotic cells in tissue culture. Microbios. 1998;96:71–93. [PubMed] [Google Scholar]