Abstract
The pathogenesis of diabetes in the nonobese diabetic (NOD) mouse is characterized by a selective destruction of the insulin-producing β-cells in the islets of Langerhans mediated by autoreactive T cells. The function of T cells is controlled by dendritic cells (DC), which are not only the most potent activators of naïve T cells, but also contribute significantly to the establishment of central and peripheral tolerance. In this study, we demonstrate that the NOD mouse (H2: Kd, Ag7, E°, Db) shows selective phenotypic and functional abnormalities in DC derived from bone marrow progeny cells in response to GM-CSF (DCNOD). NOD DC, in contrast to CBA DC, have very low levels of intracellular I-A molecules and cell surface expression of MHC class II, CD80, CD86 and CD40 but normal β2-microglobulin expression. Incubation with the strong inflammatory stimulus of LPS and IFN-γ does not increase class II MHC, CD80 or CD86, but upregulates the level of CD40. The genetic defect observed in the DCNOD does not map to the MHC, because the DC from the MHC congenic NOD.H2h4 mouse (H2: Kk, Ak, Ek, Dk) shares the cell surface phenotype of the DCNOD. DC from these NOD.H2h4 also fail to present HEL or the appropriate HEL-peptide to an antigen-specific T cell hybridoma. However all the DC irrespective of origin were able to produce TNF-α, IL‐6, low levels of IL-12(p70) and NO in response to LPS plus IFN-γ. A gene or genes specific to the NOD strain, but outside the MHC region, therefore must regulate the differentiation of DC in response to GM-CSF. This defect may contribute to the complex genetic aetiology of the multifactorial autoimmune phenotype of the NOD strain.
Keywords: Insulin dependent type I diabetes, dendritic cells, NOD mouse, antigen presentation
INTRODUCTION
The spontaneous animal model for human type I diabetes, the nonobese diabetic (NOD) mouse is characterized by a selective destruction of the insulin-producing β-cells following cellular inflammation initiated at 3–4 weeks of age by macrophages and dendritic cells followed by T and B lymphocytes [1]. Destruction of macrophages (and dendritic cells) in the NOD mouse prevents diabetes [2–4] although CD4 and CD8 T cells are believed to be the effector cells.
The disease in the NOD mouse is determined by several genes (Idd1–19) at least four of which map to the MHC region [5–8]. The non-MHC genes collectively give the animals an autoimmune-prone susceptibility genotype with the H2 alleles determining the phenotype of the autoimmune disease [9]. Exposure of the NOD mice to many different microbial agents or altering the diet can reduce or prevent diabetes demonstrating that environmental factors also play a role in the pathogenesis (see [10] and [11]). Several studies have shown that the NOD mouse has a defect in thymic selection of T cells leading to a higher level of autoreactive T cells than seen in other mouse strains [12–14]. Although this may to a degree be caused by the unusual I-Ag7 allele [15], non-MHC diabetogenic genes also contribute to the autoproliferative phenotype [14].
Dendritic cells (DC) are the most important activators of naïve T cell responses. They differentiate from bone marrow-derived stem cells, and reside in nonhaemopoeitic tissues (e.g. skin, mucosa, solid organs) as ‘immature’ DC characterized by a high ability to capture antigens through endocytosis, pinocytosis or interaction with pattern recognition receptors on the cell surface. When foreign antigen is encountered, the DC migrate towards the appropriate lymph node whilst differentiating into ‘mature’ DC characterized by high surface expression of MHC class II and costimulatory molecules and efficient antigen presentation. DC play a key role in the induction of self–tolerance. Interaction between DC and thymocytes in the thymus shapes the peripheral T cell repertoire by ensuring that only the pre-T cells able to recognize peptides in the context of self-MHC are selected, and that the thymocytes expressing T cell receptors with too high affinity for self-peptide/self-MHC are eliminated. This positive and negative selection provides the immune system with central tolerance. In addition, since not all self peptides are available for T cell selection in the thymus, interaction between DC and T cells in the secondary lymphoid organs may also establish peripheral tolerance [16].
Mouse DC can be generated in vitro by culture of bone marrow precursors in GM-CSF [17]. The NOD mouse has abnormalities in the production of myeloid progeny cells produced from bone marrow in response to GM-CSF (and IL‐3 and IL‐5) compared to that of BALB/c mice [18]. In this study, we have examined the phenotype and function of DC generated from culture of bone marrow from NOD mice. Unexpectedly, we have demonstrated that there are profound phenotypic differences in the bone marrow-derived DC from NOD mice, and these differences cannot be overcome by driving differentiation with lipopolysaccharide (LPS) and IFN-γ. This defect is controlled by gene(s) mapping outside the MHC region.
MATERIALS AND METHODS
Animals
Female NOD and NOD.H2h4 mice were kindly provided by Dr A. Cooke, Department of Pathology, Cambridge University and Professor E. Simpson, Clinical Science Centre, London. Female CBA/Ca/Olac mice were purchased from Harlan UK Ltd. The mice were used at ages from 6 to 14 weeks, and none of the autoimmune-prone animals used had developed autoimmune disease when the bone marrow was isolated.
Tissue culture
The hen egg lysozyme (HEL) specific T cell hybridoma IC5·1 (I-Ak-restricted) was maintained in RPMI medium (Gibco BRL, UK) supplemented with 10% FCS and antibiotics. Dendritic cells were obtained in vitro by growing bone marrow stem cells from the femurs in Iscove's Modified Dulbecco's Medium (IMDM) (Gibco BRL, UK) supplemented with 10% FCS (Gibco BRL, UK), 5×10−5 M 2-mercaptoethanol (2-ME) (Gibco BRL, UK), 5μl/ml Transferrin (Sigma, UK), 100 U/mlPenicillin and 100μg/ml Streptomycin (Gibco BRL, UK) and NaHCO3 (Sigma, UK) and 10% of conditioned medium from the cell line X-63 (kindly provided by B. Stockinger, NIMR, UK) containing granulocyte-macrophage colony-stimulating factor (GM-CSF). The bone marrow cells from three mice were pooled to set up a batch of cells. The DC populations obtained from all three strains contained 90–95% CD11c+ cells by FACS analysis and less than 5% CD220+ cells. For stimulation with lipopolysaccharide (LPS) and IFN-γ, the dendritic cells from a day 7 culture were harvested, washed, and 3×106 DC were incubated with 100 ng/ml LPS and 100 U/ml murine rIFN-γ for 18 h.
For antigen presentation assays DC from 7 day cultures were either stimulated with 100 ng per ml LPS and 100 U per ml IFN-γ or left unstimulated for 18 h. The cells were harvested, washed twice with MEM (Gibco BRL, UK) and resuspended in complete IMDM. Dendritic cells were plated, in triplicate, at 104 cells per well with 5 × 104 IC5·1 T hybridoma cells in a 96 well plate. Native HEL (Boehringer Mannheim, UK) or a synthetic peptide corresponding to the HEL dominant epitope (amino acid sequence 46–61 (HEL(46–61)) was added at different concentrations, and the plates were incubated for 20 h at 37°C and 5% CO2. Supernatants were collected and assayed for the presence of IL-2, by measuring the proliferation of the IL-2 dependent cell line CTLL-2. Fifty microliters of supernatant was added to 5 × 103 CTLL-2 and cells were incubated for 24 h. Cells were pulsed with 1 µCi per well of[3H]-thymidine (ICN, UK), for 18 h and then harvested. Incorporation of[3H]-thymidine was determined using a Trilux 1450 Microbeta liquid scintillation counter (Wallac). The results are shown as the mean of each triplicate.
Flow cytometry
After 18 h with or without LPS/IFN-γ stimulation the dendritic cells were harvested, washed and resuspended at 1 × 106 cells per ml. Aliquots of 1 × 105 DC were incubated with 50 μl 10% rabbit serum (Sigma, UK) in sterile HBSS (Gibco BRL, UK) containing 0·1% Sodium Azide (Sigma, UK) at 4°C for 15 min followed by addition of primary antibodies. After incubation on ice for 45 min the cells were spun at 1600 r.p.m. for 5 min at 4°C and washed twice with 100 μl HBSS before being resuspended in 100 µl HBSS. Fifty μl of the secondary antibodies was added, incubated on ice for 45 min and washed twice. The cells were finally fixed by adding 50 μl HBSS and 100 μl 3·7% formaldehyde (Sigma, UK) in PBS, and analysed by flow cytometry on a Becton Dickinson FACScan using WIN MDI software.
The antibodies used in the study were the MHC class II specific rat antimouse I-Ak clone MRC Ox-6 (Serotec, Oxford, UK) and anti-I-Ag7 clone 10·2.16 (kindly provided by EP Reich, Anergen Inc), rat antimouse CD80 (B7·1) clone IG10 (Pharmingen, San Diego, USA), rat antimouse CD86 (B7·2) clone GL1 (Cambridge Bioscience, UK); rat antimouse CD40 clone 3/23 (Serotec, UK), hamster antimouse CD11c clone N-418 (produced by ICRF, London, UK); FITC-conjugated rabbit antirat Ig (Dako, Ely, UK); FITC-conjugated rabbit anti‐mouse Ig (Dako, Denmark); and mouse anti‐mouse β2-microglobulin clone S19·8 (kindly provided by M Millrain, CSC, UK).
Intracellular staining
After 18 h without or with LPS/IFN-γ stimulation the dendritic cells from CBA or NOD mice were allowed to settle for 30 min on fibronectin-coated 5 mm diameter glass cover slips placed at 37°C. Attached cells were fixed with 4% paraformaldehyd (Sigma, UK) for 5 min followed by permealization with 0·05% saponin (Sigma, UK) in PBS containing 0·2% bovine serum albumin. The permeabilized cells were then processed for immunofluorescence. Purified rat anti‐mouse CD16/CD32 Fc blocking antibodies clone 2·4G2 (Pharmingen, USA) were added and the cells incubated for 15 min on ice. FITC-conjugated anti‐mouse I-Ak clone MRC Ox-6 (Pharmingen, USA), FITC-conjugated rat anti‐mouse class II antibodies (Cambridge Bioscience, Cambridge, UK) or isotype controls were then added, and the cells were incubated for further 45 min on ice. The cells were washed three times before the cover slips were mounted on glass slices for microscopy. The cells were viewed through an inverted Zeiss Axiovert 100 microscope in conjunction with a Bio-Rad (Hemel Hempstead, UK) Laser Scanning Confocal Imaging System.
Cytokine and NO2− assays
Dendritic cell supernatants used for determining the expression of cytokines and NO were collected after 18 h incubation of DC in the presence or absence of 100 ng/ml LPS and 100 U/ml rIFN-γ. Quantification of the cytokines was done using standard capture ELISA assays with antibody concentrations as recommended by the manufacturers and the appropriate purified recombinant cytokines as standards using flat-bottomed MaxiSorb microtitre plates (Life Technologies, Paisley, UK). The antibodies used were hamster antimouse TNF-α (Gift from Celltech, Slough, UK), rabbit anti‐mouse TNF-α (Genzyme, West Malling, UK), peroxidase-conjugated goat anti‐rabbit IgG (Sigma, Poole, UK), rat anti‐mouse IL-6 (Genzyme, USA), biotinylated rat anti‐mouse IL-6 clone MP5–32C11 (Pharmingen, USA), rat anti‐mouse IL-12 p40, clone 15·6 (Genzyme, USA) and biotinylated rat anti‐mouse IL12 (p40/70) clone C17·8 (Pharmingen, USA). Recombinant mouse cytokines TNF-α, IL‐6 and IL‐12 (p70heterodimer) were obtained from Pharmingen, USA. The concentration of the cytokines in the samples was calculated as pg per ml using the linear part of the standard curve.
NO was quantified as NO2− by incubating 100 µl aliquots of appropriately diluted medium with 100 µl per well of Griess' reagent (0·1% N-1-naphtylenediamine dihydrocloride in H2O + 1% sulphanilamide in 5% H3PO4 mixed 1 : 1) for 10 min in the dark according to Ding et al. [19].
RESULTS
Phenotype of GM-CSF expanded dendritic cells
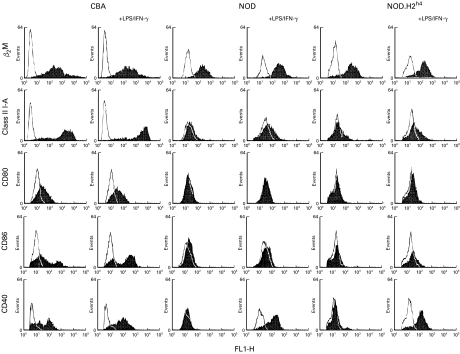
Dendritic cells were generated from NOD, NOD.H2h4 (a congenic NOD strain with the MHC haplotype Kk, I-Ak, I-Ek and Dk) and CBA mice by incubating bone marrow cell suspension in the presence of GM-CSF. Figure 1 shows the phenotype of the cell populations, which were all 90–95% positive for CD11c (results not shown). The DCCBA were I-A positive with an I-Ahi population consisting of approximately 70% of the DCCBA and an I-Alo population containing the remainder of the CD11c+ cells. The DCCBA expressed moderate amount of β2-microglobulin and CD80, and fractions of the DCCBA expressed CD86 and CD40. Stimulation with LPS – a powerful activator of the dendritic cells – and IFN-γ for 18 hours increased the surface expression of I-A (from a Gmean of 567 to a Gmean of 815) for the I-Ahi population without affecting either the size or the expression level of the I-Alo population. LPS/IFN-γ also induced the expression of CD40 on all the DCCBA and significantly increased the size of the CD86+ population. Activation with LPS/IFN-γ did not effect the cell surface levels of β2-microglobulin or CD80 considerably. Thus incubation with GM-CSF generated mainly immature DC from bone marrow of CBA mice, a fraction of which matured further in response to LPS/IFN-γ as have been reported for DC derived from other mouse strains [20,21]. This contrasted with the phenotype of the DC isolated from the NOD mouse (DCNOD) which is characterized by lack of I-A, CD86 and CD40 surface expression (Fig. 1). Even when stimulated with LPS/IFN-γ for 18 hours the DC fail to upregulate the cell surface level of I-A and CD86. Only an upregulation of CD40 expression on all the DCNOD was observed (Fig. 1).
Fig. 1.
Surface phenotype of bone marrow derived DC. The DC consisting of 90–95% CD11c+ cells were analysed by flow cytometry for the expression of I-A, β2-microglobulin, CD80, CD86 and CD40 (▪) or by staining with secondary antibody alone (□). Two I-A specific mAbs, Ox-6 (shown here) or 10·2.16 were used with identical result. The results are representative of three different experiments carried out three weeks apart.
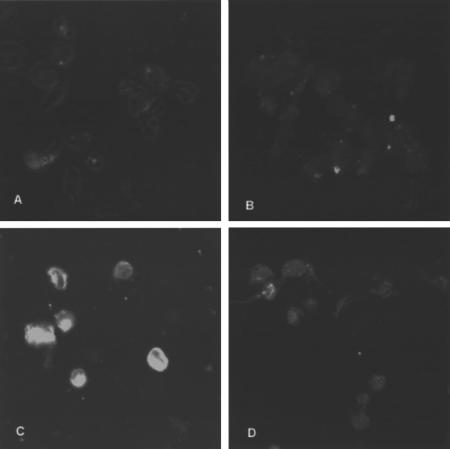
To investigate whether LPS/IFN-γ could induce a synthesis of I-A molecules that would accumulate intracellularly, we stained saponin-permeabilized DCCBA and DCNOD cells with I-A-specific antibodies. As shown in Fig. 2 very low levels of intracellular MHC class II molecules were seen in the DC isolated from both mouse strains when unstimulated. However, after incubation with LPS/IFN-γ for 18 h, a clear accumulation of MHC class II molecules were seen in the DCCBA whereas the DCNOD accumulated significantly lower levels of assembled I-A molecules in the different intracellular compartments. This suggests that the NOD mouse encode some defect, which affects the differentiation of bone marrow stem cells into immature DC in response to GM-CSF. This defect maps outside the MHC region because the DC generated from bone marrow of the MHC congenic NOD mouse, NOD.H2h4, shares the phenotype of the DCNOD (Fig. 1).
Fig. 2.
Intracellular staining of MHC class II molecules in bone marrow derived DC. Bone marrow derived DC from CBA (A and C) and NOD (B and D) without (A and B) or with (C and D) LPS/IFN-γ stimulation were permeabilized with saponin and stained with I-Ak reactive monoclonal antibody Ox-6.
Antigen presentation
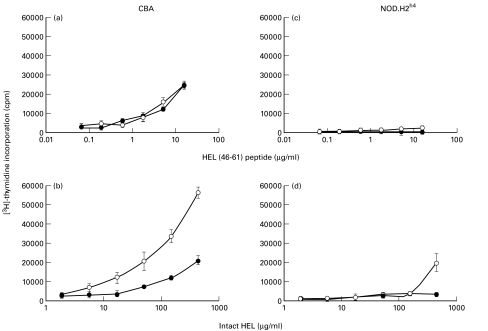
We next wanted to determine whether the observed differences in the phenotype between the CBA and NOD derived DC affected their ability to stimulate antigen-specific T cell proliferation. We utilized the fact that CBA and NOD.H2h4 share MHC class II alleles and thus could be analysed under identical conditions. The DCCBA and DCh4 were incubated with either intact hen egg lysozyme (HEL) or a peptide containing the I-Ak Th epitope, HEL(46–61), and with the I-Ak-restricted HEL(46–61)-specific T cell hybridoma IC5·1. As shown in Fig. 3a, DCCBA loaded with HEL(46–61) were able to induce IL-2 production in IC5·1 cells in a dose dependent manner irrespective of whether the DC had been stimulated with LPS/IFN-γ or not. Unstimulated DCCBA were furthermore competent to internalize and process intact HEL and promote the IC5·1 hybridoma to proliferate (Fig. 3b). Maturing DCCBA with LPS/IFN-γ reduced the antigen presenting capacity of the DC markedly demonstrating that LPS/IFN-γ mature some of the DC into a pure antigen presenting mode with limited processing abilities (Fig. 3b).
Fig. 3.
Presentation of HEL and HEL(46–61) peptide by DC to I-Ak restricted HEL-specific IC5·1 hybridomas. Increasing concentrations of the HEL(46–61) peptide (a, c) or intact HEL (b, d) were incubated with DC derived from CBA (a, b) or NOD.H2h4 (c, d), which had been stimulated for 18 h with (•) or without (○) LPS/IFN-γ. The production of IL-2 was determined using IL-2 dependent CTLL-2 cells. The result of one of two different experiments is shown.
The DC from NOD.H2h4 had a minimal ability to stimulate proliferation of IC5·1 irrespective of whether the cells were pulsed with HEL(46–61) or intact HEL (Fig. 3c,d). Only at very high concentrations of 500 µg per ml of HEL did the nonstimulated DCh4 show an appreciable stimulation of the IC5·1 cells. Even DCh4 incubated with LPS/IFN-γ and pulsed with the peptide failed to induce any IL-2 production by IC5·1. This is consistent with the very low expression of MHC class II and CD80/CD86 on the DCh4.
Cytokine expression
To further characterize the DC from the NOD and NOD.H2h4 in relation to the antigen-uptake and antigen-presenting competent DCCBA we measured the production of cytokines and NO in unstimulated and LPS/IFN-γ stimulated cells (Table 1). Very little TNF-α, IL-6, and IL-12 (p70) were produced in the unstimulated immature DC from any of the mouse strains, but stimulation with LPS/IFN-γ induced a significant upregulation of TNF-α and IL‐6 production, and a very modest expression of IL-12 (p70). The cytokine levels produced by the DC from the three different mouse strains were all comparable. No differences in NO synthesis were seen in the DC from the three different mouse strains. The NOD genotype did not therefore alter the ability of the cells derived in GM-CSF either to respond to LPS/IFN-γ or to release the three inflammatory cytokines measured.
Table 1.
Cytokine production (pg/ml) and NO release [μm] by bone marrow derived DC (1×106 DC/ml) from CBA, NOD and NOD.H2h4 mice with or without stimulation by 100 ng/ml LPS and 100 U/ml rIFN-γ for 18 hours. Three experiments with each mouse strain shown.
| CBA | NOD | NOD.H2h4 | |||||
|---|---|---|---|---|---|---|---|
| LPS/IFN‐γ | LPS/IFN‐γ | LPS/IFN‐γ | |||||
| Exp | − | + | − | + | − | + | |
| TNF-α | 1 | 2633 | 56299 | 4840 | 44909 | 2345 | 38809 |
| 2 | 798 | 55720 | 2747 | 36384 | 17918 | 40182 | |
| 3 | 3441 | 33639 | 587 | 24384 | 10212 | 53012 | |
| IL-6 | 1 | 1592 | 23043 | 854 | 16855 | 397 | 25355 |
| 2 | 38 | 21743 | 437 | 19755 | 1097 | 21968 | |
| 3 | 1324 | 18668 | <30 | 9568 | 1994 | >26000 | |
| IL-12 | 1 | <15 | 64 | <15 | 17 | <15 | 34 |
| 2 | <15 | 79 | <15 | 16 | <15 | 16 | |
| 3 | <15 | 18 | <15 | 65 | <15 | 65 | |
| NO | 1 | <4 | 30 | <4 | 54 | <4 | 64 |
| 2 | <4 | 63 | <4 | 59 | <4 | 60 | |
| 3 | <4 | 43 | <4 | 41 | <4 | 68 | |
DISCUSSION
DC are the most efficient activators of T cells, and are the only antigen presenting cells which prime naive T cells in vivo. Immature DC, generated in vitro by incubating bone marrow cells with GM-CSF, have a high rate of synthesis but relatively low cell surface expression of MHC class II molecules and coreceptors CD80/CD86 and CD40. These cells are highly efficient in taking up and processing foreign antigen, by macropinocytosis, a specialised form of endocytosis. On exposure to pathogen-derived molecules such as LPS [20,21] or to inflammatory cytokines (e.g. IFN-γ, TNF-α), immature DC can differentiate into mature DC [16]. Mature DC are characterized by a reduced ability to capture antigen, arrest of synthesis but enhanced cell surface expression of MHC class II, upregulation of ‘costimulator’ ligands on the cell surface to enhance the ability to activate T cells, and upregulation of the synthesis of a number of cytokines. These characteristics of both immature and mature DC are illustrated in our study by the DC generated from CBA mice by culture of bone marrow precursors in GM-CSF. In the absence of further stimulus, these cells express class II MHC, but only low levels of the costimulatory ligands CD40, CD80 and CD86. Following further culture in the presence of the potent combination of LPS/IFN-γ, the DC acquire much higher levels of costimulatory molecules, an increase in the level of cell surface class II MHC, but show reduced ability to process and present the model protein antigen, hen egg lysozyme.
In contrast, the cells obtained from NOD bone marrow progeny cells cultured in GM-CSF appear to be arrested at a pre-DC stage. Although they display normal expression of CD11c, they express little or no cell surface MHC class II, CD80, CD86, and CD40. Even when exposed to the strong inflammatory signal of LPS and IFN-γ, the DCNOD fail to upregulate the surface expression of either MHC class II or CD80/86 coreceptors or show extensive intracellular clustering of MHC class II molecules, suggesting a significant reduced synthesis of I-A molecules. A similar phenotype was seen on DC from the NOD congenic strain NOD.H2h4. Since these DC expressed the I-Ak molecule like those from CBA mice, the DC could be tested for their ability to stimulate cognate T cell hybridomas. As predicted from their cell surface phenotype, NOD.H2h4 DC were unable to effectively present either protein or peptide antigen to the appropriate cognate T cell, both before and after exposure to LPS/IFN-γ. DC from NOD mice did not show a global failure to respond to LPS/IFN-γ, since this stimulus increased cell surface expression of CD40 to the same levels as DCCBA, and induced normal secretion of TNF-α, IL‐6, IL-12, and NO.
The results reported here were recently supported by Lee et al. [22], who also found lower levels of MHC class II, CD80, CD86 and CD40 on DCNOD compared with bone marrow derived DC from C57BL/6 even following stimulation with LPS, IFN-γ and anti-CD40. Thus these results demonstrate that the NOD mouse has a genetic defect, which selectively blocks the expression of class II MHC, CD80 and CD86 on bone marrow derived DC precursors cultured in the presence of GM-CSF. The molecular identity of this defect has yet to be established, but the allele(s) controlling the arrest in the differentiation of the DC precursors in vitro must map outside the MHC region, since the DC generated from the NOD and the MHC congenic NOD strain NOD.H2h4 had identical phenotype.
A number of other studies have reported abnormalities in bone-marrow differentiation in NOD mice. The number of myeloid colonies forming from bone marrow cells of NOD mice in response to GM-CSF, IL-3 and IL-5 has been shown to be smaller than that from nondiabetic mouse strains [18]. NOD mice also exhibit a defect in bone marrow macrophage differentiation in response to CSF−1 [23,24], and the macrophages remain functionally immature, as assessed by an inability to secrete IL-1 [24,25]. The lack of responsiveness was attributed to the fact that CSF-1 did not upregulate c-fms (CSF-1 receptor) and Ifgr (IFN-γ receptor) expression [24].
The differentiation of DC from bone marrow of NOD mice has also been investigated by Morel and colleagues [26,27]. These authors, as in the present study, reported some abnormalities in bone marrow DC differentiation in the NOD mice. In contrast to our results, however, they did demonstrate full maturation of DC from precursors in certain circumstances. The differences between the results obtained there and our study may result from differences in culture conditions of the DC. Most of the former studies were carried out using IL4/GM-CSF differentiation rather than GM-CSF alone, for example. In addition, subtle other differences in the preparation of cells for culture, or the source of the cytokines, may have contributed to the differences observed. Even the degree of mechanical stress during DC culture and passage, which are likely to vary somewhat between different laboratories, are known to induce maturation of DC in vitro [20].
Further studies will be needed to establish whether all the different reported abnormalities in bone marrow differentiation derive from a single genetic change. Furthermore, it is still unclear whether the failure of in vitro DC differentiation demonstrated in this study is paralleled by altered DC differentiation in vivo, or is an in vitro epiphenomenon of a more fundamental molecular defect in the response of bone marrow precursors to GM-CSF. Certainly, these results do not imply that the NOD mouse is unable to produce functional DC in vivo where a multiplicity of maturation signals exist. NOD mice are immunological competent, and several groups have isolated fully differentiated DC from secondary lymphoid tissues [28–30]. Nevertheless, our results raise the intriguing possibility that NOD mice may have a selective failure in the development of a particular population of antigen presenting cells, which impairs the overall development of self-tolerance. This hypothesis is certainly supported by several studies which have demonstrated that diabetes in NOD mice can be prevented by adoptive transfer of normal DC [18,26–29]. The identification of the biochemical/molecular changes, which underlie the phenotypic defects described in this study, will provide important additional information on the molecular aetiology of this complex multifactorial disease.
Acknowledgments
We thank Dr A. Cooke, Department of Pathology, University of Cambridge and Prof E.Simpson, Clinical Science Centre, Imperial College for Science, Technology and Medicine, London for kindly providing the mice used in this study. This work was supported by grants from The British Diabetic Association, The Arthritis and Rheumatoid Council, The Wellcome Trust and from the Committee of Scientific Research (Warsaw, Poland). J.S. was recipient of stipends from Knud Højgaards Fond, Otto Bruun's Fond, William & Hugo Evers Fond and Direktør Jacob Madsens & Hustru Olga Madsens Fond.
REFERENCES
- 1.Jansen A, Homodelarche F, Hooijkaas H, Leenen PJ, Dardenne M, Drexhage HA. Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and beta- cell destruction in NOD mice. Diabetes. 1994;43:667–75. doi: 10.2337/diab.43.5.667. [DOI] [PubMed] [Google Scholar]
- 2.Hutchings PR, Simpson E, O'Reilly LA, Lund T, Waldmann H, Cooke A. The involvement of Ly2+ T cells in beta cell destruction. J Autoimmun. 1990;1:101–9. doi: 10.1016/s0896-8411(09)90018-x. [DOI] [PubMed] [Google Scholar]
- 3.Jun HS, Yoon CS, Zbytnuik L, vanRooijen N, Yoon JW. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med. 1999;189:347–58. doi: 10.1084/jem.189.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jun HS, Santamaria P, Lim HW, Zhang ML, Yoon JW. Absolute requirement of macrophages for the development and activation of beta-cell cytotoxic CD8 (+) T-cells in T-cell receptor transgenic NOD mice. Diabetes. 1999;48:34–42. doi: 10.2337/diabetes.48.1.34. [DOI] [PubMed] [Google Scholar]
- 5.Hattori M, Buse JB, Jackson RA, et al. The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science. 1986;231:733–5. doi: 10.1126/science.3003909. [DOI] [PubMed] [Google Scholar]
- 6.Lund T, Shaikh S, Kendall E, et al. RFLP analysis of the MHC class III region defines unique haplotypes for the non-obese diabetic, cataract Shionogi and the non-obese non-diabetic mouse strains. Diabetologia. 1993;36:727–33. doi: 10.1007/BF00401143. [DOI] [PubMed] [Google Scholar]
- 7.Ikegami H, Makino S, Yamato E, et al. Identification of a new susceptibility locus for insulin-dependent diabetes-mellitus by ancestral haplotype congenic mappingI. J Clin Invest. 1995;96:1936–42. doi: 10.1172/JCI118239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hattori M, Yamato E, Itoh N, et al. Cutting edge: Homologous recombination of the MHC class I K region defines new MHC-linked diabetogenic susceptibility gene(s) in nonobese diabetic mice. J Immunol. 1999;163:1721–4. [PubMed] [Google Scholar]
- 9.Wicker LS. Major histocompatibility complex-linked control of autoimmunity. J Exp Med. 1997;186:973–5. doi: 10.1084/jem.186.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabinovitch A. Immunoregulatory and cytokine imbalances in the pathogenesis of IDDM. Diabetes. 1994;43:613–21. doi: 10.2337/diab.43.5.613. [DOI] [PubMed] [Google Scholar]
- 11.Coleman DL, Kuzava JE, Leiter EH. Effect of diet on incidence of diabetes in nonobese diabetic mice. Diabetes. 1990;39:432–6. doi: 10.2337/diab.39.4.432. [DOI] [PubMed] [Google Scholar]
- 12.Kanagawa O, Martin SM, Vaupel BA, CarrascoMarin E, Unanue ER. Autoreactivity of T cells from nonobese diabetic mice: an I-A (g7)-dependent reaction. Proc Natl Acad Sci USA. 1998;95:1721–4. doi: 10.1073/pnas.95.4.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLuca D, Bluestone JA, Shultz LD, Sharrow SO, Tatsumi Y. Programmed differentiation of murine thymocytes during fetal thymus organ culture. J Immunol Methods. 1995;178:3–29. doi: 10.1016/0022-1759(94)00236-p. [DOI] [PubMed] [Google Scholar]
- 14.Ridgway WM, Ito H, Fasso M, Yu C, Fathman CG. Analysis of the role of variation of major histocompatibility complex class II expression on nonobese diabetic (NOD) peripheral T cell response. J Exp Med. 1998;188:2267–75. doi: 10.1084/jem.188.12.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acha-Orbea H, McDevitt HO. The first external domain of the nonobese diabetic mouse class II I-A beta chain is unique. Proc Natl Acad Sci USA. 1987;84:2435–9. doi: 10.1073/pnas.84.8.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 17.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone-marrow cultures supplemented with granulocyte macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langmuir PB, Bridgett MM, Bothwell ALM, Crispe IN. Bone-marrow abnormalities in the nonobese diabetic mouse. Int Immunol. 1993;5:169–77. doi: 10.1093/intimm/5.2.169. [DOI] [PubMed] [Google Scholar]
- 19.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–12. [PubMed] [Google Scholar]
- 20.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 21.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 22.Lee M, Kim AY, Kang Y. Defects in the differentiation and function of bone marrow-derived dendritic cells in non-obese diabetic mice. J Korean Med Sci. 2000;15:217–23. doi: 10.3346/jkms.2000.15.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serreze DV, Gaskins HR, Leiter EH. Defects in the differentiation and function of antigen presenting cells in NOD/Lt mice. J Immunol. 1993;150:2534–43. [PubMed] [Google Scholar]
- 24.Serreze DV, Gaedeke JW, Leiter EH. Hematopoietic stem-cell defects underlying abnormal macrophage development and maturation in NOD Lt mice – Defective regulation of cytokine receptors and Protein-Kinase-C. Proc Acad Natl Sci USA. 1993;90:9625–9. doi: 10.1073/pnas.90.20.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob CO, Aiso S, Michie SA, McDevitt HO, Acha OH. Prevention of diabetes in nonobese diabetic mice by tumor necrosis factor (TNF): similarities between TNF-alpha and interleukin 1. Proc Natl Acad Sci USA. 1990;87:968–72. doi: 10.1073/pnas.87.3.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morel PA, Vasquez AC, Feili-Hariri M. Immunobiology of DC in NOD mice. J Leukoc Biol. 1999;66:276–80. doi: 10.1002/jlb.66.2.276. [DOI] [PubMed] [Google Scholar]
- 27.Feili-Hariri M, Dong X, Alber SM, Watkins SC, Salter RD, Morel PA. Immunotherapy of NOD mice with bone marrow-derived dendritic cells. Diabetes. 1999;48:2300–8. doi: 10.2337/diabetes.48.12.2300. [DOI] [PubMed] [Google Scholar]
- 28.Clare-Salzler MJ, Brooks J, Chai A, Vanherle K, Anderson C. Prevention of diabetes in nonobese diabetic mice by dendritic cell transfer. J Clin Invest. 1992;90:741–8. doi: 10.1172/JCI115946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinomiya M, Fazle Akbar SM, Shinomiya H, Onji M. Transfer of dendritic cells (DC) ex vivo stimulated with interferon- gamma (IFN-γ) down-modulates autoimmune diabetes in non-obese diabetic (NOD) mice. Clin Exp Immunol. 1999;117:38–43. doi: 10.1046/j.1365-2249.1999.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radosevic K, Casteels KM, Mathieu C, Van Ewijk W, Drexhage HA, Leenen PJ. Splenic dendritic cells from the non-obese diabetic mouse induce a prolonged proliferation of syngeneic T cells. A role for an impaired apoptosis of NOD T cells? J Autoimmun. 1999;13:373–82. doi: 10.1006/jaut.1999.0338. [DOI] [PubMed] [Google Scholar]