Abstract
Acute allograft rejection is primarily a consequence of clonal expansion of donor-specific T cells with specificity for donor antigen. Immunosuppression current involves the administration of toxic drugs that limit lymphoproliferation, but this treatment is not antigen-specific and allows opportunistic infection. An ideal strategy would be production of donor-specific T cell tolerance in the presence of an otherwise intact and functional T cell repertoire. Methods to enhance normal apoptotic clearance of activated T cells might contribute to development of this state. This study focuses on manipulation in vitro of Fas-mediated T cell apoptosis and compares two methods to enhance the extent and kinetics for clearance of activated T cells. First, the CD4 coreceptor was cross-linked in the presence and absence of Fas-stimulation. It was found that CD4 cross-linking potently induced apoptosis, even in the absence of Fas stimulation. Resting and activated T cells were susceptible to this treatment, precluding the development of antigen-specific tolerance after T cell activation. In a second system, T cells were treated with two staurosporine analogues, Bisindolylmaleimide (Bis) III and VIII and apoptosis was induced by stimulation of Fas. Resting T cells remained resistant to Fas-mediated apoptosis, but treatment of mitogen or alloantigen-activated cells with either Bis III or VIII caused a synergistic increase in apoptosis. These agents also reduced the period of resistance to Fas-mediated apoptosis after T cell activation, possibly by reducing expression of c-FLIP, allowing early activation of caspase 8 in alloreactive T cells. Development of this strategy might provide a route to the induction of specific tolerance after organ transplantation.
Keywords: tolerance, T cell apoptosis, Fas, FLIP, bisindolylmaleimide
Introduction
In transplantation the ideal form of immunosuppression would induce specific tolerance or deletion of donor antigen-reactive T cells, negating the hazards currently associated with chronic, broad-spectrum immunosuppressive therapy. A scenario such as this has been hypothesized to explain the spontaneous acceptance of liver allografts by some patients [1], and may also account for the maintenance of immune privilege in the anterior chamber of the eye and the testis [2,3]. These examples of spontaneous immune tolerance are all associated with an extensive induction of localized T cell apoptosis [1,4].
The population of antigen-specific effector T cells produced by clonal expansion after initial antigen-encounter is programmed to decline when it becomes redundant. This is achieved by two mechanisms that both produce T cell apoptosis. The first is described as passive cell death [5] and occurs when an activated T cell is deprived of the cytokines necessary for its survival [6]. The second is termed activation-induced cell death (AICD) [7], which is produced in sensitive cells by interaction between cell-surface death receptors of the TNF superfamily and their corresponding ligands on adjacent cells. The best-characterized death receptor is Fas (CD95 or APO-1) and it is through this receptor that AICD is predominantly mediated [8]. The importance of AICD for immune homeostasis is illustrated by the lymphadenopathy and autoimmunity produced in lpr mice, which carry a mutant Fas protein [9]; humans expressing defective Fas suffer a similar pathology termed Canale–Smith syndrome [10].
In a previous study, our group has shown that induction of the apoptosis of donor-antigen specific T cells can produce a measure of specific immune hyporesponsiveness to re-challenge with donor cells [11]. Significantly, T cells demonstrate resistance to Fas-mediated apoptosis for the first 5 days after activation, presumably to allow effector function to occur, but then show an increasing sensitivity to AICD [12,13]. The balance between pro- and anti-apoptotic proteins within the activated T cells must explain this time course since cell-surface expression of Fas is up-regulated rapidly after T cell activation but does not then alter greatly between early and late stages of the immune response [14]. Several studies have suggested the importance of an anti-apoptotic protein FLIP (FLICE-inhibitory protein) for the regulation of AICD [15,16]; FLICE, or caspase 8, is a primary effector of the cascade resulting in Fas-mediated apoptosis. Cellular FLIP exists as numerous splice variants at the mRNA level, but only two forms, termed FLIPL and FLIPS, exist at the protein level [17]. These proteins are expressed at high levels in freshly activated T cells but expression declines after 6 days, providing a potential explanation for enhanced sensitivity to Fas-mediated apoptosis [15].
Stimulation of Fas clearly provides a route to apoptotic deletion of antigen-specific T cells after organ transplantation. However, the resistance of cells to this approach for at least 5 days following activation provides a sufficient period to allow tissue damage to occur; indeed, in the absence of other immunosuppression many organs in experimental transplant models will lose function within this time [18]. Several studies have focused on techniques to accelerate the kinetics for induction of T cell apoptosis. For example, it has been shown that apoptosis can be enhanced by cross-linking the CD4 coreceptor on T cells [19,20]. Significantly, this mechanism might provide an explanation for the prolongation of graft survival produced in some animal transplant models by administration of anti-CD4 antibodies [21,22].
It has also been reported recently that the extent of Fas-mediated apoptosis of T cells can be enhanced by treatment of the cells with Bisindolylmaleimide (Bis) VIII, an analogue of the protein kinase C inhibitor staurosporine [23]. This agent has been used successfully to potentiate apoptosis of auto-antigen reactive T cells in multiple sclerosis and experimental allergic encephlomyelitis (EAE) [23,24]. In the latter disease, Bis VIII produced a significant amelioration of neurological signs. One possible explanation for the activity of Bis VIII is suggested by the observation that a further Bis derivative (Bis III) can down-regulate FLIP expression in dendritic cells, leading to increased sensitivity to Fas-mediated apoptosis [25].
In this study we have investigated the potential of CD4 cross-linking and of treatment of T cells with either Bis III or Bis VIII to reduce the period of resistance to Fas-mediated apoptosis following T cell activation; a range of model systems including resting and mitogen activated peripheral T cells, the Jurkat T cell line, and alloantigen-activated T cells were investigated. In a further series of experiments we examine the effect of Bis III and Bis VIII on the expression of cellular FLIP and active caspase 8 in a Fas-stimulated, mixed lymphocyte reaction model.
Materials and methods
Cell culture
The human Jurkat T cell line (J16) and its Fas-resistant form (J16-R) were kindly provided by Professor Peter Krammer, German Cancer Research Centre, Heidelburg [26], and grown in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 U/ml penicillin and 100 µg/ml streptomycin (all from Life Technologies, Paisley, UK). The cells were split routinely and maintained at fewer than 1 × 106 cells/ml to ensure minimal levels of background apoptosis. Peripheral blood mononuclear cells were obtained from healthy volunteers by dilution of heparinized venous blood and centrifugation over Ficoll-hypaque density gradients (ρ = 1·077; Lymphoprep; Nycomed UK, Birmingham, UK) for 25 min at 400 g. The mononuclear cells were harvested from the interface, washed once by centrifugation at 400 g for 8 min, resuspended in medium to 1 × 106 cells/ml and allowed to adhere to 75 cm2 tissue culture flasks (Corning; High Wycombe, UK) at 37°C for 2 h. The non-adherent peripheral blood lymphocytes (PBL) were decanted, adjusted to 1 × 106 cells/ml and used in subsequent experiments, these cells typically contained fewer than 2% monocytes.
T cell activation
CD3 cross-linking
The anti-CD3 antibody OKT-3 (Janssen-Cilag, High Wycombe, UK) was diluted in PBS to an optimal concentration of 5 µg/ml and 200 µl aliquots were added to each well of a 24-well plate (Corning) for 2 h at 37°C. The wells were then washed three times with PBS and once with RPMI 1640 medium. After washing, 2 ml of PBL were added to each antibody coated well and incubated for varying times at 37°C.
Mixed lymphocyte reaction (MLR)
An EBV-transformed B cell line was generated as described previously [27] and used as the stimulator population in all one-way MLRs; before use further proliferation of the B cell line was prevented by γ-irradiation (50 Gy; 137Cs source). Stimulator cells were mixed with allogeneic responder PBL at a responder to stimulator cell ratio of 2 : 1. Typically, batch MLRs were carried out in tilted 25 cm2 flasks in a total volume of 10 ml. Lymphocytes were harvested from the reaction at various time points for experimental use.
Induction of apoptosis
Cells were treated with anti-Fas antibody (clone DX-2; BD Pharmingen, Heidelburg, Germany) at a previously determined optimum concentration of 5 µg/ml. In some assays CD4 was cross-linked by incubation of PBL or CD3 activated PBL with 5 µg of anti-CD4 antibody (clone RPA-T4; BD Pharmingen) for 1 h at 4°C; this was controlled by use of an isotype-matched irrelevant antibody (BD Pharmingen). The wells of a 24-well plate (Corning) were coated with 200 µl aliquots of an 80-µg/ml stock of goat antimouse antibody (GAM; BD Pharmingen) for 2 h at 37°C before washing. The CD4-labelled PBL or CD3 activated PBL were washed and added to the GAM coated plates for 1 h at 37°C. Following this incubation period the cells were harvested, washed and adjusted to a concentration of 2 × 106 cells/ml. One ml of cells was then transferred to each well of a 24-well plate (Corning) and incubated at 37°C for 18 h. A further series of assays included either Bis III or Bis VIII (both from Alexis Biochemicals, San Diego, USA) at an optimal concentration of 10 µm. Staurosporine (Sigma, Poole, UK) was used at 5 µm to induce positive control apoptosis.
Detection of cell death
Annexin V analysis of cell death
Annexin V-FITC (Sigma) was used at 20 ng/ml in binding buffer (10 mm HEPES, 150 mm NaCl, 5 mm KCl, 1 mm MgCl2, 1·8 mm CaCl2), all from Sigma. Briefly, 1 × 105 cells were centrifuged at 200 g for 5 min and resuspended in 200 µl of annexin V-FITC in binding buffer. The samples were then incubated at 37°C for 15 min and analysed by fluorescence flow cytometry.
DNA staining: assessment of DNA fragmentation
Cells were centrifuged at 500 g for 5 min and washed once with PBS and resuspended in a buffer consisting of Isoton (Beckman Coulter, High Wycombe, UK), 5% (v/v) Triton X-100 (Sigma), and 2·5 µg/ml propidium iodide (Sigma). Samples were incubated at 4°C in the dark for 1 min and analysed immediately by flow cytometry for quantification of percentage of lymphocytes showing a hypodiploid staining profile. Specific apoptosis was expressed following subtraction of the percentage of apoptotic cells in non-induced control cell populations; background typically did not exceed 10%.
Protein extraction and immunoblotting
Cells were treated as indicated in the text and whole cell protein extracts were prepared in lysis buffer (50 mm Tris, pH 7·4, 150 mm NaCl, 0·2 mm Na3VO4, 1% NP-40, 1 mm PMSF, 1 mm DTT, 25 µg/ml each of Leupeptin, Aprotinin and Pepstatin; Sigma), on ice with gentle agitation for 30 min. Cellular debris was removed by centrifugation. Protein concentrations were determined using the bicinchoninic acid method (Pierce, IL, USA) using BSA (Sigma) as a standard curve. Following denaturation by incubation at 95°C for 5 min, 20 µg of protein per lane was separated by 10% SDS-PAGE [28]. Protein was transferred to a nitrocellulose membrane (Hybond P; Amersham Pharmacia Biotech, Little Chalfont, UK) by electroblotting overnight at 4°C. The membrane was blocked with 5% non-fat milk powder (Marvel; Premier Beverages, Stafford, UK) in Tris buffered saline (TBS) for 1 h and was incubated with a polyclonal anti-FLIP antibody (Alexis Biochemicals). Subsequent washes were carried out in TBS followed by incubation with a goat antirabbit secondary antibody conjugated to horseradish peroxidase (HRP) (BD Transduction Laboratories, USA). Antibody binding was detected using an enhanced chemiluminescence system (Amersham Pharmacia Biotech). Subsequent blot stripping, secondary probing and development to identify the ‘housekeeping’ protein α-tubulin (Sigma) was used routinely to control for equal protein loading. For graphical quantification of protein expression band density was integrated after scanning with molecular analyst software (Biorad, Hertfordshire, UK)
Analysis of caspase 8 activity
The ‘Apoalert’ caspase-8 colourimetric assay kit (Clontech, Palo Alto, USA) was used to assess caspase 8 activity in lymphocytes harvested from an MLR at a range of time points. Briefly, 3–5 × 106 cells were centrifuged at 200 g for 5 min. The pellets were resuspended with 50 µl of chilled lysis buffer provided and incubated on ice for 10 min. Cell lysates were pelleted at 10 000 g for 1 min and the supernatants were recovered and either stored at −70°C or kept on ice. Typically, 100 µg of total protein were used in each well of the assay, this was predetermined by the bicinchoninic acid method (Pierce). The assay was read at 405 nm and analysed using Revelation Quicklink software (Dynax Technologies, Ashford, Middlesex, UK).
Statistical analysis
Each experiment was performed on at least three occasions; typically, three replicates were established in each assay. The significance of the data was determined using Student's t-test (Minitab software; CleCom Ltd, Birmingham, UK).
Results
Induction of apoptosis by cross-linking CD4 on resting and activated lymphocytes
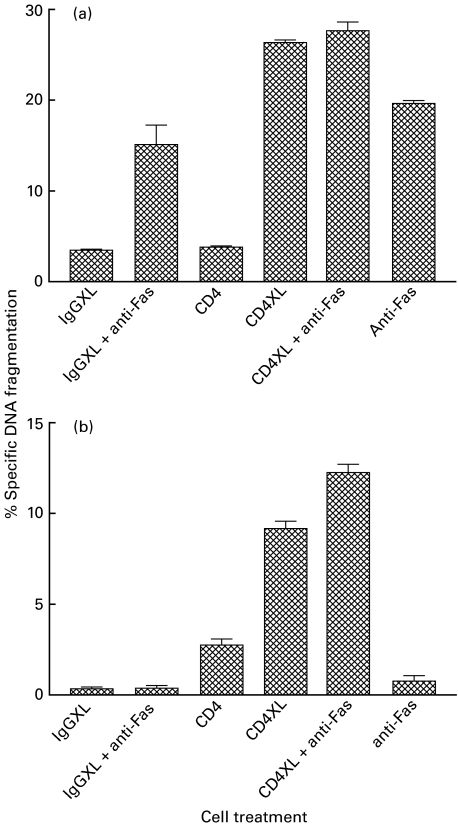
Following T cell activation for 3 days by cross-linking CD3, it was found that stimulation with anti-Fas produced a small but significant increase in apoptosis (P < 0·0001; Fig. 1a). At this time after T cell activation, stimulation with anti-CD4 produced no significant increase in apoptosis but subsequent cross-linking produced more apoptosis (P < 0·0001; Fig. 1a) than was generated by antibody stimulation of Fas. Combination of cross-linking CD4 and stimulation of Fas produced no more apoptosis than was generated by cross-linking CD4 alone (P > 0·05; Fig. 1a).
Fig. 1.
CD4 cross-linking induces non-specific apoptosis in activated and unactivated lymphocytes. PBLs activated by immobilized anti-CD3 for 3 days (a) or fresh PBLs (b) were cross-linked (XL) with an anti-CD4 antibody for 1 h at 4°C followed by cross-linking with an immobilized goat antimouse antibody and incubation with or without an anti-Fas antibody for 24 h. An isotype matched antibody was used for control (IgG). Cell death was quantified by analysis of the hypodiploid population. The experiment was perfomed three times with similar results; the mean and s.e.m. of the data are shown.
In a separate series of experiments it was found that freshly isolated PBL were resistant to the induction of apoptosis following stimulation with anti-Fas (Fig. 1b). However, addition of anti-CD4 alone produced a small increase in apoptosis that was greatly increased by CD4 cross-linking (P < 0·01). Combination of cross-linking CD4 and antibody-stimulation of Fas produced only a small additional increase in apoptosis (P < 0·01). Irrelevant, isotype-matched IgG antibodies were used either with or without the application of subsequent cross-linking for control in all these experiments; the failure of these reagents to induce significant apoptosis demonstrated the specificity of the experimental system.
Examination of the toxicity of Bis III and Bis VIII
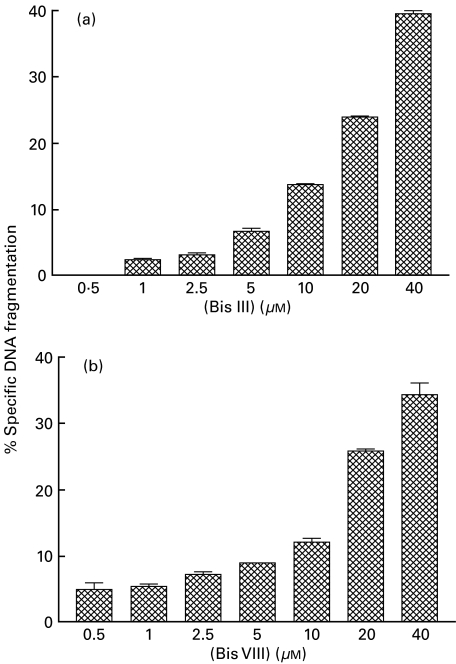
The data shown in Fig. 2 shows the effect of treatment of Jurkat T cells for 18 h with varying concentrations of either Bis III (Fig. 2a) or Bis VIII (Fig. 2b). Neither drug produced significant apoptosis of the indicator T cell line when used at low concentrations. Use of the drugs at 10 µm produced only minimal apoptosis but higher concentration resulted in significant cell death (P < 0·001; P < 0·002, respectively). Toxicity titrations were also carried out on anti-CD3 activated T cells, giving a similar toxicity profile (results not shown). For these reasons, both drugs were used at a concentration of 10 µm in all subsequent experiments.
Fig. 2.
The Bis derivatives have cytotoxic effects on Jurkat cells. Jurkat T cells were treated with a variety of concentrations of Bis III (a) or Bis VIII (b) for 18 h. Apoptosis was measured by propidium iodide determination of the hypodiploid population. The experiment was performed twice with similar results; the mean and s.e.m. of the data are shown.
Examination of the potentiation of Fas-mediated apoptosis by addition of Bis III or Bis VIII to Jurkat cells
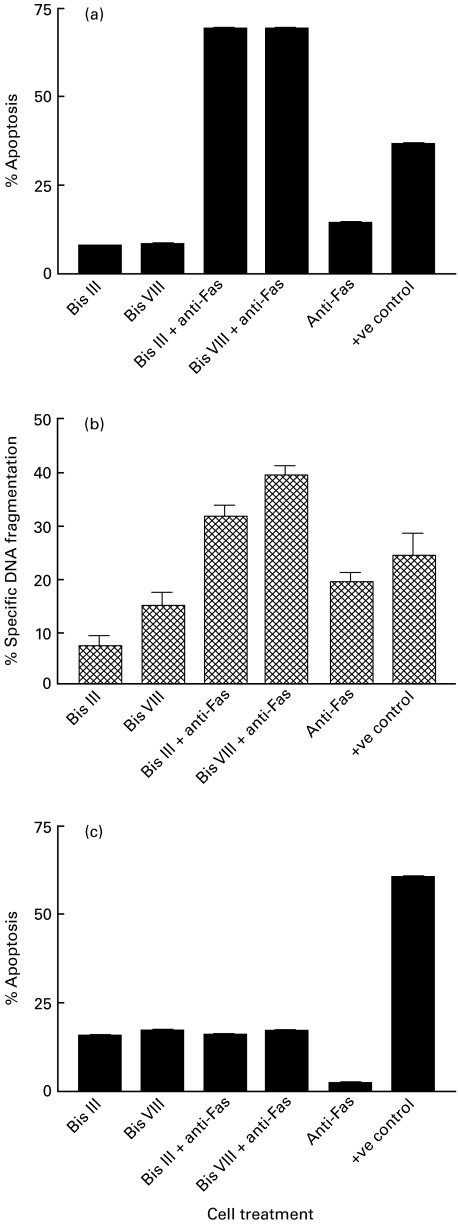
To determine whether Bis III or Bis VIII modify the susceptibility of Fas-sensitive Jurkat T cells to induction of apoptosis, these cells were incubated with a combined treatment of either Bis III or Bis VIII at a concentration of 10 µm and anti-Fas antibody at 5 µg/ml. Apoptosis was measured after 18 h either by annexin V binding (Fig. 3a) or by PI quantification of the hypodiploid cell population (Fig. 3b). A slight increase in apoptosis was observed following addition of the Bis derivatives alone, but this was most probably caused by the slight toxicity of these drugs at 10 µm. It was also found that antibody stimulation of Fas alone produced a significant but relatively small apoptotic response. However, supplementation with either Bis III or Bis VIII greatly enhanced the apoptosis induced by Fas-ligation (P < 0·0001 and P < 0·0001, respectively; Fig. 3a). For control, a Fas-resistant Jurkat cell line (J16-R) which lacks cell-surface Fas was treated in an identical way to the Fas-sensitive cell line. These cells showed no evidence of enhanced Fas-mediated apoptosis either in the presence or absence of the Bis derivatives (Fig. 3c).
Fig. 3.
Bis derivatives potentiate Fas-mediated apoptosis in a Fas-sensitive but not a Fas-resistant Jurkat cell line. J16 and J16-R cell lines were treated with 10 μm BisIII/Bis VIII with or without anti-Fas for 18 h. The positive control used was 5 μm staurosporine. Apoptosis was detected by both annexin V-FITC binding (a and c), and measurement of the hypodiploid population (b) as determined by FACS analysis. Experiments were carried out in triplicate. Results are presented as mean and s.e.m. of one representative experiment of four performed. Percentage apoptosis was calculated as described in Materials and methods.
Examination of the role of T cell activation in Bis-enhanced, Fas-mediated apoptosis
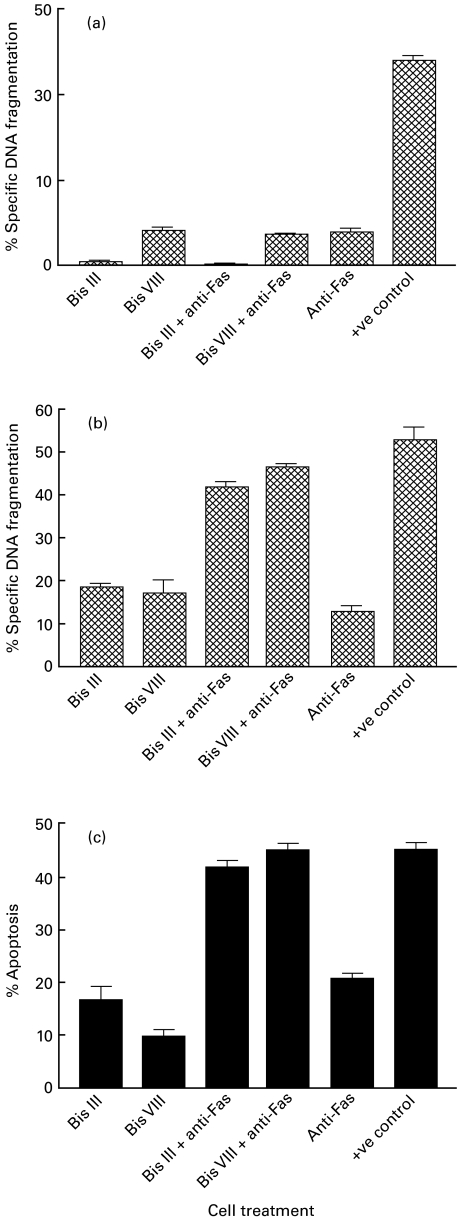
Following definition of conditions for co-administration of Bis derivatives and anti-Fas using the Jurkat T cell line, a series of experiments was established to examine the result of treatment of resting and anti-CD3 activated T cells. It was found that resting PBL were resistant to Fas-mediated apoptosis and that addition of either Bis III or Bis VIII to this system produced no additional cell death (Fig. 4a). However, as before (Fig. 1a), it was found that T cells which had been activated with anti-CD3 for 3 days were sensitive to Fas mediated apoptosis but the amount of apoptosis was greatly enhanced by additional stimulation with Bis III or Bis VIII, as determined by PI detection of the subdiploid population (P < 0·0001 and P < 0·0001, respectively; Fig. 4b) and annexin V analysis (P < 0·0001 and P < 0·0001, respectively; Fig. 4c). It is noteworthy that similar sets of data were obtained from the two techniques used for the detection of apoptosis.
Fig. 4.
Fas-mediated apoptosis is enhanced in anti-CD3 activated lymphocytes by Bis derivatives but not in resting PBLs. Freshly isolated PBLs (a) and PBLs activated through anti-CD3 activation (b and c) were incubated for 18 h with either medium alone, 10 μm Bis III/Bis VIII, 5 μg/ml of anti-Fas antibody or co-incubation with Bis III/Bis VIII and anti-Fas. Staurosporine (5 μm) was used as a positive control. Apoptosis was measured by either PI detection of the hypodiploid population (a and b) or annexin V binding (c). The experiment was carried out four times with similar results; the mean and s.e.m. of the data are shown.
Examination of the mechanism by which Bis derivatives enhance Fas-mediated T cell apoptosis
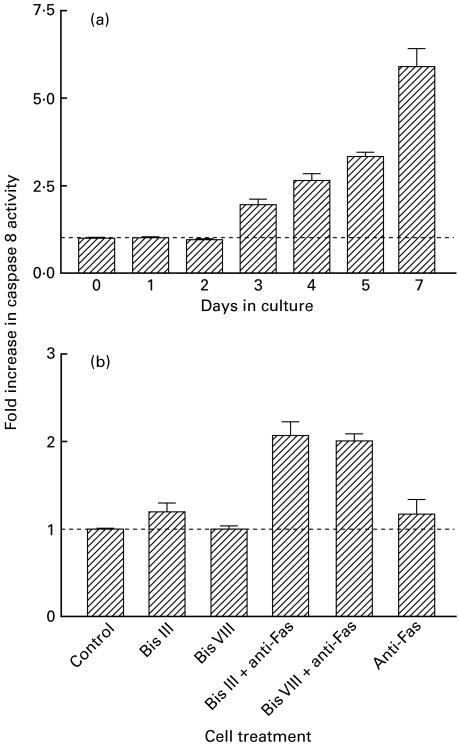
It is known that induction of apoptosis by ligation of Fas on activated T cells is critically dependent on the activation of caspase 8. A series of experiments was designed to measure the potential to induce caspase 8 activation by stimulation of Fas on T cells harvested at serial time points from an in vitro model of the graft rejection response, the MLR. Despite high level expression of cell-surface Fas (data not shown) it was found that high levels of active caspase 8 were only detected in cells taken from an MLR after culture for at least 5 days (Fig. 5a). However, following supplementation of the assay system with either Bis III or Bis VIII, it was found that increased levels of active caspase 8 were generated after culture periods as brief as 2 days (Fig. 5b).
Fig. 5.
Caspase 8 activity increases with stage of activation of T cells and can be augmented by treatment with Bis III and Bis VIII. In vitro caspase 8 activation assays were carried out on lysates of cells from MLRs at the time points indicated (a). Cells from day 2 MLRs were incubated with either medium, Bis III/Bis VIII (10 μm) or anti-Fas (5 μg/ml) alone, or co-incubated with both Bis III/Bis VIII and anti-Fas (b). The experiment was performed three times with similar results; the mean and s.e.m. of the data are shown. Results are expressed as the fold increase in caspase 8 activity (experimental/control).
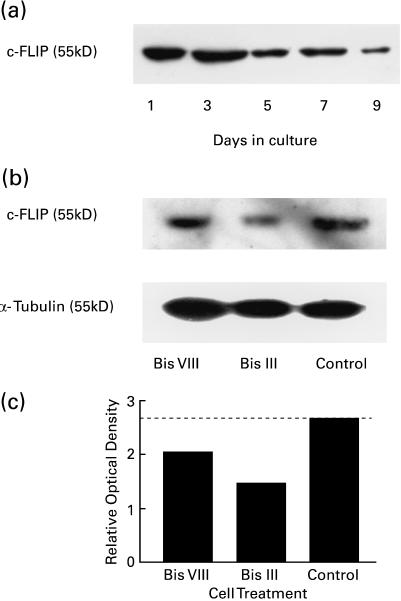
Parallel experiments were performed to quantify the expression of cellular FLIP, a protein that is known to inhibit the activation of caspase 8. In this study it was shown that FLIP expression declines gradually with time in an MLR, showing a marked reduction after 5 days (Fig. 6a). Significantly, after 2 days in culture it was found that addition of either Bis III or Bis VIII for 18 h caused a reduction in FLIP expression at a time when the expression of this protein by untreated control cells from the same culture remained high (Fig. 6b, c).
Fig. 6.
FLIP expression in activated T cells can be reduced by Bis treatment. PBLs activated in an MLR were sampled at the time points indicated and the expression of the 55 kD protein c-FLIP was assessed by Western blotting analysis of lysates (a). Day 2 MLRs were treated with Bis III or Bis VIII (10 μm) and the expression of c-FLIP was determined by Western blotting (b). Equal protein loading was determined by reprobing for α-tubulin (b: lower blot). The relative changes in the expression of c-FLIP were determined by densitometry (c).
Discussion
It is becoming increasingly clear that functional removal of donor antigen specific T cells represents a realistic strategy for the induction of long-term graft tolerance following organ transplantation [29]. While this might be achieved by mechanisms including the induction of regulatory T cells and the generation of antigen-specific T cell anergy following blockade of costimulation, an attractive possibility remains apoptotic deletion of donor-specific T cells. Indeed, there is some evidence to suggest that intragraft T cell apoptosis might contribute to the spontaneous acceptance of liver allografts observed in animal models and after some clinical transplant procedures [1].
The possibility that stimulation of Fas on the surface of donor-specific T cells can produce specific graft tolerance was first explored by Bellgrau et al. [3], who showed that Fas ligand (FasL)-expressing testicular allografts were accepted after transplantation, while grafts from FasL-deficient donor animals were normally rejected. However, later papers have shown that the expression of FasL is not always sufficient to generate long-term allograft survival [30]. Indeed, it has been shown that inappropriate expression of FasL can promote neutrophil-mediated rejection, although this may be overcome by the administration of TGFβ [31].
A significant problem for the generation of graft tolerance, through the induction of apoptosis of activated T cells, remains the period of resistance to Fas-mediated apoptosis observed after primary T cell activation [12]. While this interval is necessary for effective T cell immune functions to take place, it is also sufficient to allow significant tissue damage to occur after organ transplantation. In order to produce donor specific tolerance, a strategy must be developed which spares resting, non-donor reactive T lymphocytes but potentiates the kinetics for apoptotic clearance of allospecific T cells.
It has been reported that cross-linking the CD4 co-receptor with specific antibody can augment the apoptosis of T lymphocytes [32]. Indeed, this mechanism has been proposed to account for the widespread deletion of CD4+ T cells observed in patients infected with the CD4-binding virus, HIV-1 [20]. In both cases it has been shown that stimulation of CD4 results in increased cell-surface expression of Fas, while blockade of this signalling system can prevent apoptotic T cell deletion. However, there remains some confusion surrounding the consequence for T cells of CD4 binding. Several groups have suggested that ligation of CD4 can prime resting T cells for AICD [19,32], while others have suggested that CD4 cross-linking can actually inhibit this process [33,34]. Despite this controversy, treatment with non-depleting anti-CD4 antibodies is well known to provide a route to specific graft tolerance in some murine transplant models [22,35].
In the current study, the induction of Fas-mediated apoptosis was compared between activated and resting T cells following CD4 cross-linking in order to determine the potential of this treatment to enhance early deletion of activated allospecific T cells while sparing the remaining T cell repertoire. Following T cell activation by cross-linking CD3 for 3 days it was found that stimulation with anti-Fas produced an apoptotic response, but this was markedly smaller than that elicited by cross-linking CD4. These two stimuli were neither additive nor synergistic, as combined stimulation of both receptors produced no more apoptosis than cross-linking CD4 alone.
In common with previous studies, it was found that resting T cells were resistant to the induction of apoptosis by stimulation of Fas. However, a large apoptotic response was observed following CD4 cross-linking, with evidence of a small additional increase produced by simultaneous stimulation of both Fas and CD4 cross-linking; this observation is consistent with previous studies [36]. These results indicate that cross-linking CD4 provides a powerful pro-apoptotic stimulus. Furthermore, it was shown that resting T cells become vulnerable to apoptosis after only a brief period of CD4 cross-linking, suggesting accelerated kinetics of the normal AICD mechanism [36]. However, the potential to cause apoptosis of resting cells also suggests that this mechanism is likely to produce generalized immunosuppression, rather than the specific deletion of donor reactive cells required for graft tolerance. Therefore, organ tolerance achieved following anti-CD4 monoclonal antibody therapy [35] is potentially caused by mechanisms that do not involve the apoptotic deletion of the allospecific cells.
A further approach to enhance AICD has been suggested recently by Zhou et al. [23], who used the staurosporine analogue, Bis VIII to augment the sensitivity of T cells to Fas-mediated apoptosis. In addition, application of this compound ameliorated disease in the autoimmune models EAE and adjuvant arthritis [23]. Since allograft rejection is mediated by an analogous response to antigen expressed within an organ graft, it is possible that a similar therapeutic strategy will prove useful.
A series of in vitro models was developed to examine the apparent synergy between Fas-mediated apoptosis and treatment with Bis derivatives. Initially, it was found that the apoptotic response of a Fas-sensitive Jurkat T cell line was greatly enhanced by stimulation of Fas in the presence of either Bis III or Bis VIII. This process was confirmed to be Fas-dependent by demonstration that a Fas-resistant Jurkat cell line [26] showed no evidence of enhanced apoptosis following the addition of anti-Fas antibodies in the presence of either of the Bis derivatives. This series of experiments demonstrated that the Bis compounds could enhance the amount of Fas-mediated T cell apoptosis, but provided no information about the kinetics of acquisition of sensitivity to this form of apoptosis after initial T cell activation.
Significantly, it was found that resting T cells showed little apoptosis in the presence of either Bis III or Bis VIII; furthermore, these Bis-treated cells remained resistant to stimulation with anti-Fas suggesting that, unlike the situation with CD4 cross-linking, this strategy will not produce non-specific death of resting T cells. The resistance of resting T cells to Fas-mediated apoptosis in the presence of the Bis derivatives may be due simply to the relatively low-level expression of Fas on these cells. However, it has also been suggested that resting lymphocytes show a ‘type II’ phenotype with respect to apoptosis, resulting in failure to assemble the Fas-associated death-inducing signalling complex (DISC) together with protective over-expression of Bcl-xL [15].
Following T cell activation for 3 days by CD3 cross-linking, the cells showed a relatively small apoptotic response following addition of the agonistic anti-Fas antibody; the limited extent of apoptosis at this time point is consistent with results published previously [37]. However, stimulation of Fas in the presence of either Bis III or Bis VIII produced a significant increase in cell death. These data suggest that the Bis derivatives increase the sensitivity of T cells to Fas-mediated apoptosis at early time points after activation.
In this part of the current study, two methods were used to assess apoptosis: identification of phosphatidyl serine (PS) on the outer leaflet of the plasma membrane by annexin V staining, and measurement of the release of fragmented DNA. In the presence of the Bis derivatives and anti-Fas antibody, both methods produced similar results indicating maturation of the apoptotic response from early transmembrane PS migration through to DNA fragmentation. This contrasts with the observation of Wendling et al. [24], who demonstrated annexin V binding and caspase activation in the absence of DNA fragmentation in autoantigen-reactive T cells stimulated with Bis VIII and soluble FasL.
The mechanism by which Bis derivatives enhance Fas-mediated apoptosis is unclear. However, a study of the potentiating effect of Bis III on Fas-mediated apoptosis of otherwise Fas-resistant dendritic cells has implicated down-regulation of the anti-apoptotic protein FLIP [25]. Several studies have suggested that FLIP can also play a role in protecting activated T cells from Fas-mediated apoptosis [17]. Indeed, a FLIP (casper)-deficient mouse model has allowed definition of the function of FLIP in blockade of caspase 8 activation [38], which is an important early event in the Fas-signalling pathway [39].
Caspase 8 is the initiating caspase in Fas-mediated apoptosis in type I cells [40]. Upon binding of FasL to the Fas receptor, clustering of the receptor occurs followed by aggregation of the Fas associated death domain (FADD) and pro-caspase 8 molecules to form the DISC [41]. According to the induced proximity model, conditions within the DISC result in activation of the pro-caspase 8 zymogen to active caspase 8 [42]. However, recruitment of the caspase homologue FLIP to the DISC results in the inhibition of caspase 8 activation [15]. A previous study has shown that T cells activated by contact with alloantigens in a mixed leucocyte culture maintain a high level of FLIP for the first 5 days after activation [13], this period coincides with resistance to Fas-mediated apoptosis and failure of caspase 8 activation.
In the current study it was shown that treatment with either Bis III or Bis VIII at concentrations capable of augmenting Fas-mediated apoptosis markedly reduced the level of FLIP in T cells harvested 2 days after alloantigen-specific activation. At this time point it was also found that stimulation of Fas resulted in greater caspase 8 activation in Bis-treated cells than in untreated cells. A similar pattern was observed from day 1 to day 4, but as the basal level of caspase activity increased at day 3, so the effect of the Bis derivatives on Fas-mediated apoptosis was less pronounced (results not shown). These observations suggest that treatment of T cells with either Bis III or Bis VIII can reduce the period of resistance to Fas-mediated apoptosis following antigen-specific activation.
The results presented here suggest the possibility of accelerating the kinetics and extent of Fas-mediated apoptosis as a strategy to delete the T cells that respond to alloantigen after organ transplantation and mediate graft rejection. Since activated cells are targeted specifically, this may offer a potential strategy for early removal of donor-specific T cells, resulting in immune tolerance of a functioning transplanted organ. However, it is necessary to determine first if apoptotic deletion of allospecific T cells will compromise the immune response by also removing cross-reactive, pathogen specific lymphocytes.
Acknowledgments
Financial support for this work was provided by the Northern Counties Kidney Research Fund.
REFERENCES
- 1.Sharland A, Yan Y, Wang C, et al. Evidence that apoptosis of activated T cells occurs in spontaneous tolerance of liver allografts and is blocked by manipulations which break tolerance. Transplantation. 1999;68:1736–45. doi: 10.1097/00007890-199912150-00018. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan HJ, Streilein JW. Immune response to immunisation via the anterior chamber of the eye. I. F1 lymphocyte-induced immune deviation. J Immunol. 1977;118:809–14. [PubMed] [Google Scholar]
- 3.Bellgrau D, Gold D, Selawry H, et al. A role for CD95 ligand in preventing graft-rejection. Nature. 1995;377:630–2. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 4.Meyer D, Baumgardt S, Loeffeler S, et al. Apoptosis of T lymphocytes in liver and/or small bowel allografts during tolerance induction. Transplantation. 1998;66:1530–6. doi: 10.1097/00007890-199812150-00018. [DOI] [PubMed] [Google Scholar]
- 5.VanParijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243–8. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 6.Akbar AN, Salmon M. Cellular environments and apoptosis: tissue microenvironments control activated T-cell death. Immunol Today. 1997;18:72–6. doi: 10.1016/s0167-5699(97)01003-7. [DOI] [PubMed] [Google Scholar]
- 7.Brunner T, Mogil RJ, Laface D, et al. Cell-autonomous Fas (CD95) Fas–ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–4. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 8.Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–74. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 9.Nagata S, Suda T. Fas and Fas ligand – lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 10.Drappa J, Vaishnaw AK, Sullivan KE, et al. Fas gene mutations in the Canale–Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. N Engl J Med. 1996;335:1643–9. doi: 10.1056/NEJM199611283352204. [DOI] [PubMed] [Google Scholar]
- 11.O'Flaherty E, Ali S, Pettit SJ, et al. Examination of the sensitivity of T cells to Fas ligation. Transplantation. 1998;66:1–7. doi: 10.1097/00007890-199810270-00017. [DOI] [PubMed] [Google Scholar]
- 12.Owen-Schaub LB, Yonehara S, Crump WL, et al. DNA fragmentation and cell death is selectively triggered in activated human lymphocytes by Fas antigen engagement. Cell Immunol. 1992;140:197–205. doi: 10.1016/0008-8749(92)90187-t. [DOI] [PubMed] [Google Scholar]
- 13.O'Flaherty E, Wong WK, Pettit SJ, et al. Regulation of T-cell apoptosis: a mixed lymphocyte reaction model. Immunology. 2000;100:289–99. doi: 10.1046/j.1365-2567.2000.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Miller R, ZhangJ G. Characterisation of apoptosis-resistant antigen-specific T cells in vivo. J Exp Med. 1996;183:2065–73. doi: 10.1084/jem.183.5.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scaffidi C, Schmitz I, Krammer PH, et al. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem. 1999;274:1541–8. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- 16.Kirchhoff S, Muller WW, Krueger A, et al. TCR-mediated up-regulation of c-FLIPshort correlates with resistance toward CD95-mediated apoptosis by blocking death-inducing signaling complex activity. J Immunol. 2000;165:6293–300. doi: 10.4049/jimmunol.165.11.6293. [DOI] [PubMed] [Google Scholar]
- 17.Irmler M, Thome M, Hahne M, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–5. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 18.Kirby JA, Reader JA, Parfett GJ, et al. Rat heterotopic heart transplantation. Quantification and analysis of cell mediated cytotoxicity. Clin Exp Immunol. 1988;71:113–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Choy EHS, Adjave J, Forrest L, et al. Chimaeric anti-CD4 monoclonal antibody crosslinked by monocyte Fcγ receptor mediates apoptosis of human CD4 lymphocytes. Eur J Immunol. 1993;23:2676. doi: 10.1002/eji.1830231043. [DOI] [PubMed] [Google Scholar]
- 20.Banda NK, Bernier J, Kurahara DK, et al. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J Exp Med. 1992;176:1099–106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall BM, Fava L, Chen J, et al. Anti-CD4 monoclonal antibody-induced tolerance to MHC-incompatible cardiac allografts maintained by CD4+ suppressor T cells that are not dependent upon IL-4. J Immunol. 1998;161:5147–56. [PubMed] [Google Scholar]
- 22.Motoyama K, Arima T, Yu S, et al. The kinetics of tolerance induction by nondepleting anti-CD4 monoclonal antibody (RIB 5/2) plus intravenous donor alloantigen administration. Transplantation. 2000;69:285–93. doi: 10.1097/00007890-200001270-00015. [DOI] [PubMed] [Google Scholar]
- 23.Zhou T, Song L, Yang P, et al. Bisindolylmaleimide VIII facilitates Fas-mediated apoptosis and inhibits T cell-mediated autoimmune diseases. Nat Med. 1999;5:42–8. doi: 10.1038/4723. [DOI] [PubMed] [Google Scholar]
- 24.Wendling U, Aktas O, Schmierer K, et al. Partial synergy of bisindolylmaleimide with apoptotic stimulus in antigen-specific T cells-implications for multiple sclerosis. J Neuroimmunol. 2000;103:69–75. doi: 10.1016/s0165-5728(99)00214-3. 10.1016/s0165-5728(99)00214-3. [DOI] [PubMed] [Google Scholar]
- 25.Willems F, Amraoui Z, Vanderheyde N, et al. Expression of c-FLIP (L) and resistance to CD95-mediated apoptosis of monocyte-derived dendritic cells: inhibition by bisindolylmaleimide. Blood. 2000;95:3478–82. [PubMed] [Google Scholar]
- 26.Peter ME, Dhein J, Ehret A, et al. Apo-1 (CD95)-dependent and -independent antigen receptor-induced apoptosis in human T and B cell lines. Int Immun. 1995;7:1873–7. doi: 10.1093/intimm/7.11.1873. [DOI] [PubMed] [Google Scholar]
- 27.Wilson JL, Cunningham AC, Kirby JA. Alloantigen presentation by B cells. Analysis of the requirement for B cell activation. Immunology. 1995;86:325–30. [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–9. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Wood KJ. New concepts in tolerance. Clin Transplant. 1996;10:93–9. [PubMed] [Google Scholar]
- 30.Allison J, Georgiou HM, Strasser A, et al. Transgenic expression of CD95 ligand on islet beta cells induces a granulocytic infiltration but does not confer immune privilege upon islet allografts. Proc Natl Acad Sci USA. 1997;94:3943–7. doi: 10.1073/pnas.94.8.3943. 10.1073/pnas.94.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang S-M, Lin Z, Ascher NL, et al. Fas ligand expression on islets as well as multiple cell lines results in accelerated neutrophilic rejection. Transplant Proc. 1998;30:538. doi: 10.1016/s0041-1345(97)01396-1. [DOI] [PubMed] [Google Scholar]
- 32.Desbarats J, Freed JH, Campbell PA, et al. Fas (CD95) expression and death-mediated function are induced by CD4 cross-linking of CD4+ T cells. Proc Natl Acad Sci USA. 1996;93:11014–8. doi: 10.1073/pnas.93.20.11014. 10.1073/pnas.93.20.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberg H-H, Sanzenbacher R, Lengl-Janssen B, et al. Ligation of cell surface CD4 inhibits activation induced death of human T lymphocytes at the level of Fas-ligand expression. J Immunol. 1997;159:5742–9. [PubMed] [Google Scholar]
- 34.Sanzenbacher R, Kabelitz D, Janssen O. SLP-76 binding to p56lck. A role for SLP-76 in CD4-induced desensitization of the TCR/CD3 signaling complex. J Immunol. 1999;163:3143–52. [PubMed] [Google Scholar]
- 35.Pearson TT, Darby CR, Bushell AR, et al. The assessment of transplantation tolerance induced by anti-CD4 monoclonal antibody in the murine model. Transplantation. 1993;55:361–7. doi: 10.1097/00007890-199302000-00025. [DOI] [PubMed] [Google Scholar]
- 36.Algeciras A, Dockrell DH, Lynch DH, et al. CD4 regulates susceptibility to Fas ligand- and tumor necrosis factor-mediated apoptosis. J Exp Med. 1998;187:711–20. doi: 10.1084/jem.187.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Algeciras-Schimnich A, Griffith TS, Lynch DH, et al. Cell cycle-dependent regulation of FLIP levels and susceptibility to Fas-mediated apoptosis. J Immunol. 1999;162:5205–11. [PubMed] [Google Scholar]
- 38.Yeh WC, Itie A, Elia AJ, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–42. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- 39.Peter ME, Kischkel FC, Scheuerpflug CG, et al. Resistance of cultured peripheral T cells towards activation-induced cell death involves a lack of recruitment of FLICE (MACH/caspase 8) to the CD95 death-inducing signaling complex. Eur J Immunol. 1997;27:1207–12. doi: 10.1002/eji.1830270523. [DOI] [PubMed] [Google Scholar]
- 40.Scaffidi C, Schmitz I, Zha J, et al. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999;274:22532–8. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- 41.Kischkel FC, Hellbardt S, Behrmann I, et al. Cytotoxicity-dependent Apo-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (Disc) with the receptor. Embo J. 1995;14:5579–88. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salvesen GS, Dixit VM. Caspase activation; the induced-proximity model. Proc Natl Acad Sci USA. 1999;96:10964–7. doi: 10.1073/pnas.96.20.10964. 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]