Apoptosis is a highly regulated pathway of cell death that is critically important in development, homeostasis and some disease processes. Recent data from our laboratory and others suggest that apoptosis may have a dual role after transplantation, one of graft destruction during rejection and a beneficial role involving the deletion of alloreactive lymphocytes. In fact, there is an increasing body of evidence to suggest that apoptosis is essential to the induction of tolerance to an allograft. Transplantation of murine liver grafts results in tolerance, yet even in the absence of rejection the liver grafts are rapidly infiltrated by T cells of recipient origin [1]. Furthermore, a significant proportion of the graft-infiltrating cells in tolerant grafts are apoptotic in contrast to the infiltrating cells detected in liver isografts or allografts induced to undergo rejection by administration of IL-2. Follow-up studies indicate that IL-12 antagonism suppresses rejection by restoring apoptosis of the peripheral alloreactive T cell population [2]. Similarly in rat models of liver and liver/small bowel transplantation, tolerance is associated with apoptosis in the lymphocyte infiltrate [3]. Administration of donor leucocytes induces long-term acceptance of MHC mismatched rat liver or kidney transplants. In these models there was increased expression of IL-2 and evidence for apoptosis of recipient T cells within the graft [4].
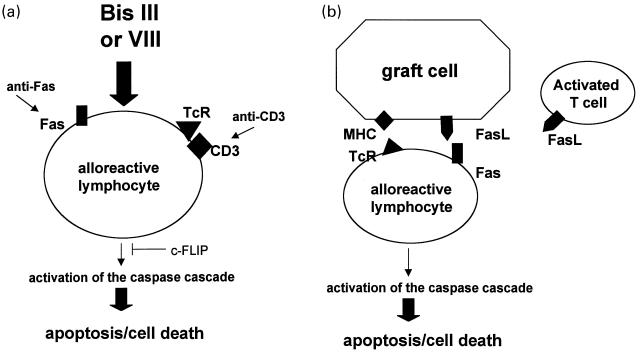
In this issue Carroll and colleagues describes a novel way of enhancing apoptosis of activated lymphocytes which may prove useful in inducing allograft tolerance [5]. They demonstrate that the staurosporine analogues Bisindolylmaleimide (Bis) III and VIII increase the sensitivity of anti-CD3 activated T cells to Fas-mediated apoptosis (Fig. 1a). Importantly they further demonstrate that resting lymphocytes are refractory to this treatment. Further studies using animal models are necessary to address whether Bis can specifically induce apoptosis of graft-reactive T cells. It should be noted that Bis VIII did prevent the development of autoimmune symptoms in rodent models of experimental allergic encephalitis and adjuvant arthritis [6].
Fig. 1.
Approaches to eliminating graft reactive lymphocytes. a, The staurosporine analogues Bis III and VIII increase the sensitivity of activated T cells to caspase-dependent Fas-mediated apoptosis. b, Elimination of alloreactive lymphocytes via Fas-mediated cell death. Graft cells or activated T cells, which either naturally express FasL or are genetically engineered to express FasL, can engage Fas on alloreactive T cells and trigger caspase-dependent apoptosis.
The best studied apoptotic pathway involves the interaction of the TNF receptor superfamily member Fas with its ligand (FasL) [7,8]. Fas/FasL interactions are important in T cell mediated cell death and constitutes a key peripheral immunoregulatory mechanism that limits the expansion of antigen-activated T cells.
FasL is expressed on some immune-privileged tissue, including the anterior chamber of the eye and testis [9,10], and thus has prompted speculation that the power of the Fas pathway could be harnessed to promote the deletion of alloreactive cells. Clearly the transplantation of allogeneic corneas is clinically successful. Similarly, in experimental models the transplantation of allogeneic FasL-expressing Sertoli cells of the testes are tolerated while testes from allogeneic, FasL-deficient gld mice, are promptly rejected [9]. Co-transplantation of allogeneic islets with allogeneic testicular cells prolonged graft survival in a rat model [11]. These studies suggest that FasL expression on target tissue could induce apoptosis of invading lymphocytes and therefore protect from an immune attack (Fig. 1b). Similarly, genetically engineered, FasL+ myoblasts were shown to prolong allograft survival when cotransplanted with allogeneic islets [12]. Prolonged graft survival was observed in rat recipients of allogeneic kidney perfused with murine FasL-expressing adenovirus [13]. There was prolonged liver allograft survival in rats that received allogeneic FasL-transfected livers. Thus in several models FasL-expressing cells can induce apoptosis of locally encountered effector T cells. Furthermore, recent data suggest that genetically engineered expression of FasL on cells could control the human T cell response towards allogeneic tissue [14].
However, expression of FasL to specifically delete alloreactive cells is likely not to achieve the ‘holy grail’ of tolerance as there are numerous reports indicating that elevated FasL expression results in graft damage and rejection. When transgenic mice which express FasL in the islet β cells were transplanted into mice the allogeneic FasL-expressing grafts developed a granulocytic infiltrate and were rejected [15]. Similarly, islet cells transduced in vitro with an adenoviral vector expressing FasL were rapidly infiltrated by neutrophils and underwent accelerated rejection [16]. Accelerated rejection of heart grafts was also observed when hearts from FasL-transgenic mice were transplanted into syngeneic or allogeneic mice [17]. Taken together, these studies suggest that local over-expression of FasL may in fact promote neutrophil accumulation and rejection. Although the precise mechanism leading to the granulocytic infiltration has yet to be elucidated, it is possible that FasL is a chemoattractant for neutrophils [18]. Thus the proinflammatory effects of FasL have to be mitigated before this novel approach will be useful in promoting tolerance. As a step in this direction, it has been demonstrated that FasL-transfected tumour cells injected into the intraocular space are not rejected due to the presence of TGF-β which inhibits neutrophil activation [19]. These studies suggest that coadministration of this cytokine with FasL-transfected cells may promote immunologic tolerance.
Thus the report by Carroll et al. [5] is important as it describes a novel strategy with the potential for inducing apoptosis of alloreactive cells. Since in their study activated T cells undergo Fas-mediated apoptosis earlier after activation, the numbers of T cells with the potential to develop effector function would be reduced. Although this scenario may prove effective in deleting alloreactive T cells in a graft, in order to promote tolerance all newly maturing alloreactive T cells would have to be eliminated. Clearly it would be desirable to spare a potential regulatory population yet eliminate the alloreactive cells.
Interestingly, one of the most commonly utilized immunosuppressive drugs given to patients who receive organ transplants has been shown to block the induction of tolerance. Blocking both CD28-B7 and CD40–CD40 ligand interactions, commonly known as costimulation blockade, results in a cell cycle dependent apoptosis of proliferating T cells along with permanent engraftment of cardiac allografts in experimental models. However, when cyclosporin A is administered along with costimulation blockade the grafts are rejected and apoptosis of T cells is abolished [20]. It is interesting to note that rapamycin, a newer immunosuppressive drug, actually increased apoptosis of alloreactive cells during costimulation blockade.
The ideal scenario post-transplant would involve a short-term drug-based or biological strategy that would lead to the specific incapacitation of alloreactive lymphocytes and tolerance to the allograft. Current immuosuppressive regimens fail on both of these counts. Tolerance is not achieved thus life-long immunosuppression is a reality for most transplant recipients. Further since these drugs target all T lymphocytes the patient is left vulnerable to infectious complications. Studies that examine new approaches towards the deletion of graft reactive T cells are clearly warranted if we are to realize the goal of achieving graft tolerance in the clinical setting.
REFERENCES
- 1.Qian S, Lu L, Fu F, et al. Apoptosis within spontaneously accepted mouse liver allografts. J Immunol. 1997;158:4654–61. [PMC free article] [PubMed] [Google Scholar]
- 2.Li W, Lu L, Wang Z, Wang L, Fung JJ, Thomson AW, Qian S. IL-12 antagonism wnhances apoptotic death of T cells within hepatic allografts from Flt3 ligand-trated donors and promotes graft acceptance. J Immunol. 2001;166:5619–28. doi: 10.4049/jimmunol.166.9.5619. [DOI] [PubMed] [Google Scholar]
- 3.Meyer D, Baumgardt S, Loeffeler S, et al. Apoptosis of T lymphocytes in liver and/or small bowel allografts during tolerance induction. Transplantation. 1998;66:1530–6. doi: 10.1097/00007890-199812150-00018. [DOI] [PubMed] [Google Scholar]
- 4.Yan Y, Shastry S, Richards C, et al. Posttransplant administration of donor leukocytes induces long-term acceptance of kidney or liver transplants by an activation-associated immune mechanism. J Immunol. 2001;166:5258–64. doi: 10.4049/jimmunol.166.8.5258. [DOI] [PubMed] [Google Scholar]
- 5.Carroll HP, Ali S, Kirby JA. Accelerating the induction of Fas-mediated T cell apoptosis: a strategy for transplant tolerance. Clin Exp Immunol. 2001;126:588–596. doi: 10.1046/j.1365-2249.2001.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou T, Song L, Yang P, et al. Bisindolylmaleimide VIII facilitates Fas-mediated apoptosis and inhibits T cell-mediated autoimmune diseases. Nat Med. 1999;5:2–48. doi: 10.1038/4723. [DOI] [PubMed] [Google Scholar]
- 7.Itoh N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–43. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 8.Oehm A, Berhmann I, Falk W, et al. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992;267:10709–15. [PubMed] [Google Scholar]
- 9.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–2. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 10.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 11.Korbutt GS, Elliot JF, Rajotte RV. Cotransplantation of allogeneic islets with allogeneic testicular aggregates allows long-term graft survival without systemic immunosuppression. Diabetes. 1997;46:317–22. doi: 10.2337/diab.46.2.317. [DOI] [PubMed] [Google Scholar]
- 12.Lau HT, Yu M, Fontana Jr A, Stoeckert CJ. Prevention of allograft rejection with engineered myoblasts expressing FasL in mice. Science. 1996;273:109–12. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- 13.Swenson KM, Ke B, Wang T, et al. Fas Ligand gene transfer to renal allografts in rats. effects on allograft survival. Transplantation. 1998;65:155–60. doi: 10.1097/00007890-199801270-00002. [DOI] [PubMed] [Google Scholar]
- 14.Dulat HJ, von Grumbkow C, Baars W, Schroder N, Wonigeit K, Schwinzer R. Down-regulation of human alloimmune responses by genetically engineered expression of CD95 ligand on stimulatory and target cells. European J Immunol. 2001;31:2217–26. doi: 10.1002/1521-4141(200107)31:7<2217::aid-immu2217>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Allison J, Georgiou HM, Strasser A, Vaux DL. Transgeneic expression of CD95 ligand on islet β cells induces a granulocytic infiltration but does not confer immune privilege upon islet allografts. Proc Natl Acad Sci. 1997;94:3943–7. doi: 10.1073/pnas.94.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang S-M, Schneider DB, Lin Z, et al. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nature Med. 1997;3:738–43. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi T, Ueki T, Nishimatsu H, et al. Accelerated rejection of Fas ligand-expressing heart grafts. Jounal Immunol. 1999;162:518–22. [PubMed] [Google Scholar]
- 18.Ottonello L, Tortolina G, Amelotti M, Dallegri F. Soluble Fas ligand is chemotactic for human neutrophilic polymorphonuclear leukocytes. J Immunol. 1999;162:3601–6. [PubMed] [Google Scholar]
- 19.Chen JJ, Sun Y, Nabel GJ. Regulation of the proinflammatory effects of Fas ligand. Science. 1998;282:1714–7. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TN. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nature Med. 1999;5:1298–302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]