Abstract
Nitric oxide (NO) plays diverse roles in physiological and pathological processes. During immune and inflammatory responses, for example in asthma, NO is generated at relatively high and sustained levels by the inducible form of nitric oxide synthase (NOS-2). NOS-2 derived NO regulates the function, growth, death and survival of many immune and inflammatory cell types. In the case of mast cells, NO suppresses antigen-induced degranulation, mediator release, and cytokine expression. The action of NO on mast cells is time dependent, requiring several hours, and noncGMP mediated, most probably involving chemical modification of proteins. NO inhibits a number of mast cell-dependent inflammatory processes in vivo, including histamine mediated vasodilatation, vasopermeation and leucocyte-endothelial cell attachment. In human asthma and animal models of lung inflammation the role of NO is harder to define. However, although there are conflicting data, the balance of evidence favours a predominantly protective role for NO. Mimicking or targeting NO dependent pathways may prove to be a valuable therapeutic approach to mast cell mediated diseases.
Keywords: allergy, cytokines, inflammation, mast cells, nitric oxide
INTRODUCTION
Nitric oxide (NO) plays diverse roles in biological systems: it is a mediator of vasodilatation, platelet aggregation and neurotransmission, and regulates function, death and survival of various cell types including many of those involved in immunity and inflammation [1–3]. In the immune system NO probably evolved as a toxic molecule in innate defence, but, in mammals at least, its role extends to immune regulation [1–3]. In the last 10 years there has been a steep rise in research directed at understanding the role and mechanisms of action of NO in immunity and inflammation. This article will introduce NO as a biologically active molecule, and then focus on NO as a regulator of mast cell activation and mast cell-dependent inflammatory processes.
NITRIC OXIDE – CHEMICAL AND BIOLOGICAL PROPERTIES
The multiple biological actions of NO are dictated by its physico-chemical properties. NO is a small (30 Da) uncharged molecule that carries an unpaired electron, thus defining it as a radical. Because of its size and absence of charge, NO diffuses unimpeded into and out of cells, and between cellular compartments. Its solubility and diffusion properties resemble closely those of oxygen, and like oxygen it is a gas under atmospheric conditions but a solute in biological systems. For a radical NO is relatively stable – it does not react with itself and has a physiological half-life of several seconds to minutes depending on its concentration and physico-chemical environment [4–6].
In response to physiological stimulation, for example in endothelial cells and neurones, NO is generated rapidly and transiently at low (picomolar) concentrations. NO so generated produces fast and transient responses in target cells such as smooth muscle cells, neurones and platelets [1,2]. In these systems NO acts directly on guanylyl cyclase to induce the second messenger cyclic guanosine monophosphate (cGMP). By contrast, in immune and inflammatory responses, cytokines and/or bacterial lipopolysaccharide induce expression of the inducible form of NO synthase (NOS-2) leading to relatively slow, sustained and high level production of NO [1–3]. Here, the actions of NO are largely indirect, being mediated by reactive nitrogen oxide species (RNOS) of formula NOx that are generated in the presence of molecular oxygen. RNOS are unstable and rapidly S-nitrosate cellular thiols including proteins, cysteine and glutathione that represent key targets in cell regulation [4–6]. These S-nitrothiols provide a slow-release storage depot for NO, hence considerably extending its biological half-life. In cells and tissues complex equilibria are set up in which NO dissociates and re-associates with thiols, at the same time freeing up NO or RNOS. This process of shunting of NO from one thiol to another, known as transnitrosation, is important in NO transport, for example from cytoplasm to nucleus [7]. Under combined nitrosative and oxidative stress, NO interacts with superoxide anion (O2−) to generate the toxic and highly reactive peroxynitrite anion (ONOO−) [4,5]. The induction and regulation of NO synthesis in the immune system, and the subsequent reactions of NO are summarized in Fig. 1. In biological systems N2O3 is the major RNOS formed from NO, and this and other RNOS are ultimately hydrolysed and excreted as nitrite (NO2−) or nitrate (NO3−) [5]. The oxidative, nonenzymatic chemical reactions of NO, including RNOS, superoxide and peroxynitrite generation, are summarized in Fig. 2.
Fig. 1.

NO synthesis and reactions in immune and inflammatory cells. In response to inflammatory cytokines or bacterial lipopolysaccharide (LPS), the enzyme NOS-2 is expressed in many cell types to produce a sustained and high level production of NO. The NO is oxidized to RNOS of generic formula NOx. These nitrosate the thiol group in glutathione to produce S-nitrosoglutathione (GS-NO) or thiol groups in proteins to generate protein-S-NO. Under oxidative stress, NO interacts with superoxide (O2−) to produce the toxic peroxynitrite anion (OONO−).
Fig. 2.
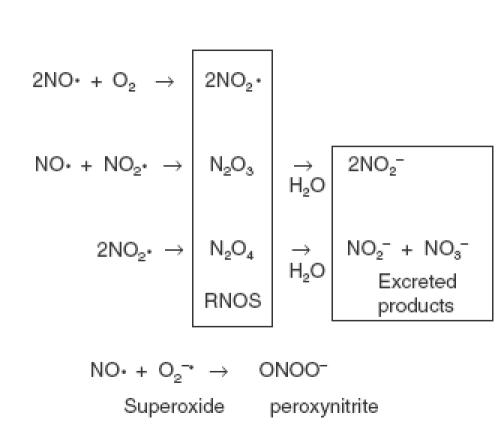
The oxidative reactions and fate of NO.
SYNTHESIS OF NITRIC OXIDE IN IMMUNE CELLS
Many cell types involved directly or indirectly in immunity and inflammation synthesize NO. These include fibroblasts, endothelial and epithelial cells, keratinocytes and chondrocytes [8,9], monocytes/macrophages [10–12], antigen presenting cells [13], natural killer (NK) cells [14], eosinophils [15] and mast cells [16,17]. Whether T lymphocytes [13,18–21], or neutrophils [22] produce biologically significant NO is still subject to some debate.
In living systems NO is synthesized from l-arginine and molecular oxygen by a process utilizing electrons donated by NADPH. The reaction is catalysed by the nitric oxide synthase (NOS) family of enzymes that convert l-arginine to NO and l-citrulline via the intermediate N-hydroxy-l-arginine [23]. One molecule of l-arginine produces one molecule of NO, the nitrogen atom of the latter deriving from the guanidino group of the arginine side chain. There are three members of the NOS family – these enzymes comprise large and complex polypeptide homodimers, and uniquely among mammalian enzymes incorporate an electron-generating reductase domain as well as an electron-receiving oxidative domain [23,24]. Two NOS are constitutively expressed (NOS-1 or neuronal NOS and NOS-3 or endothelial NOS) while the other (NOS-2 or inducible NOS) is expressed only upon cell activation. It is NOS-2, originally described in mouse macrophages [10,11], that is the major enzyme for NO synthesis in immunity and inflammation [1,2,8,9]. Unlike NOS-1 and -3, NOS-2 is not present in resting cells but is expressed following cell activation. The NOS-2 gene, which is under control of the transcription actor NFκB, is induced by bacterial polysaccharide or classical pro-inflammatory cytokines such as IL-1, tumour necrosis factor (TNF) and IFN-γ, often acting in synergy (Fig. 1). Hence expression of NOS-2 and NO production are associated with inflammatory conditions in which these cytokines are produced, or during bacterial infection [1,2,8,9]. There is a lag phase of several hours between cell activation and NO production, reflecting the time taken for mRNA and protein synthesis [23,24]. Expression of NOS-2 is inhibited by the anti-inflammatory cytokines IL-4 and IL-13, and by glucocorticoids (Fig. 1) [25].
NOS-2 is unique among the NOS family in that it is not activated by a calcium signal but is continuously active once expressed. It incorporates a calmodulin binding site to which the calmodulin is tightly bound independently of a calcium signal – this is thought to be responsible for the continuous activity of the enzyme [23,24]. Other factors, such as substrate and cofactor availability may influence NOS-2 activity. In contrast to NOS-1 and NOS-3, NOS-2 generates high concentrations of NO (nanomolar rather than picomolar) and this level of synthesis is sustained for hours or days or longer, depending on how long the enzyme is present in the cells or tissue.
MAST CELLS IN ALLERGY AND INFLAMMATION
Mast cells are highly specialized secretory cells distributed widely throughout the tissues, particularly in proximity to blood vessels, nerves and epithelial surfaces. As reviewed elsewhere [26–28] they play important roles in specific and innate immunity, and IgE-mediated allergy and inflammation. Mast cells are primed or ‘sensitised’ to respond to allergen by acquisition of IgE that binds tightly to Fc receptors. Thus, in contrast to lymphocytes, mast cells are responsive to multiple antigens, their spectrum of specificity being dictated by the repertoire of the IgE response. In IgE-mediated responses mast cells are the first cell to respond to allergen.
The cytoplasm of mast cells is packed throughout with dense granules that act as high-concentration storage sites for histamine, heparin and proteases. Within seconds or minutes of exposure to allergen, which cross-links cell surface specific IgE, mast cells expel their cytoplasmic granules and contents by exocytosis. Histamine, alone or in synergy with other mediators, contributes to the core features of inflammation, namely blood vessel dilatation and permeation, tissue swelling, raised temperature, pain and irritancy [26–28].
In addition to preformed mediators, mast cells synthesize and release certain prostaglandins and leukotrienes that in concert contribute to the immediate inflammatory response. As a consequence of mast cell activation other inflammatory cells are recruited and activated, and a cascade of inflammatory mediator production and release is set in motion. The cysteinyl leukotrienes formed through the lipoxygenase pathway of arachidonic acid oxidation are potent bronchoconstrictors and inducers of mucus secretion and contribute substantially to airway narrowing in asthma [29].
Additionally, mast cells are a rich source of diverse cytokines that are synthesized de novo upon cell activation, or also, as is the case for TNF, stored ‘ready-to-go’ in the secretory granules [30–32]. In fact mast cells are the major cell type to store TNF and are thus primed to trigger most rapidly TNF mediated inflammatory responses [33]. The list of cytokines and chemokines released from mast cells upon IgE dependent and IgE independent activation is extensive, but those that have received most attention include TNF, IL-4 and IL-6. Mast cell-derived cytokines have been implicated as the missing link between the acute and chronic stages of IgE-mediated inflammation [32]. For example, mast cell TNF is a prime candidate for promoting the later phases of inflammation by recruiting other inflammatory cell types [33].
EFFECTS OF NITRIC OXIDE ON MAST CELL ACTIVATION
Just over 10 years ago Vane and colleagues first reported an inhibitory action of NO on histamine release from rat peritoneal mast cells [34,35]. They showed that the NO donor sodium nitroprusside (NaNP) inhibited degranulation in response to the mast cell chemical activators calcium ionophore A23187 and compound 48/80 [34], and that a NOS inhibitor enhanced LPS-induced histamine release [35]. Subsequently NaNP was shown to inhibit anti-IgE-induced histamine and tryptase release from human skin mast cells [36] and anti-IgE- and calcium ionophore-induced histamine release from human basophils and rat peritoneal mast cells [37]. In another study, inhibition of NOS increased allergen-induced histamine release from isolated guinea pig heart, and this was associated with exacerbated cardiac anaphylaxis seen as decreased coronary blood flow and induction of arrhythmias [38]. Furthermore, NaNP reduced histamine release and the severity of cardiac anaphylaxis. Thus, in this heart model, NO exerts a protective effect on allergen-induced anaphylaxis, presumably through stabilization of mast cells [38].
For some years it has been known that IFN-α/β and -γ inhibit whereas IL-4 enhances IgE/antigen-induced degranulation and mediator release from mouse and rat mast cells, and ionomycin-induced cytokine mRNA induction in human HMC-1 mast cells [39–44]. The IFN-γ effect is far stronger in mixed peritoneal cells compared to purified mast cell preparations, suggesting an indirect effect of the cytokine [45]. Further experiments revealed that the active intermediate is nitric oxide – the IFN-γ effect was blocked by NOS inhibition and mimicked by NaNP and S-nitrosoglutathione (GS-NO) [45]. The IFN-γ effect was confirmed as indirect since it was seen only when the accessory cells but not the mast cells in mixed populations expressed the IFN-γ receptor [46]. Consistent with a role for NO, the enhancing effect of IL-4 on mast cells in mixed peritoneal populations correlates to inhibition of NO synthesis [47]. The NO effect on mast cells is direct since it is seen equally in mixed and purified populations of mast cells [45,47].
Some workers have claimed that NO is without regulatory activity on mast cells [48,49] but their studies have used inappropriate conditions, particularly incubation times with sources of NO. NO production or NOS expression by rat peritoneal or mouse bone marrow-derived cultured mast cells have been reported, indicating an auto-regulatory role for NO [16,17,35,50–52]. However, we have found that removal of mast cells from mixed rat and mouse peritoneal cell populations dramatically depletes NO production, and that residual NO production can be fully accounted for by the low numbers (1–2%) of contaminating nonmast cells [45,47]. Furthermore, NOS inhibitors enhance antigen-induced degranulation of mast cells in IFN-γ stimulated mixed but not purified mast cell populations, suggesting that, even if NO is produced at low levels by mast cells in response to IFN-γ, it has no functional autocrine activity in this setting [45,47]. However, in other circumstances, such as stimulation by antigen [50] or adhesion [51] mast cells may produce significant NO.
Given the interest in mast cells a source of functionally important cytokines [30–32], we have recently turned our attention to the role of NO in regulation of mast cell cytokine expression. Using the rat RBL-2H3 mast cell line as model, we have found that a panel of NO donors, of varying chemical type and half-life, each produced a time-dependent, and in some cases reversible, inhibition of IgE/antigen-induced expression of mRNA for TNF, IL-4 and IL-6 Davis et al. submitted for publication]. As can be seen from Fig. 3, GS-NO completely blocked the induced cytokine mRNA response after preincubation times of 2–4h before antigen challenge. We had shown previously that NO donors inhibit release of serotonin (a marker of degranulation) from rat and mouse peritoneal mast cells only after prolonged incubation times of up to 24h before antigen challenge [45,47]. This ‘slow’ responsiveness of cultured as well as primary mast cells to NO is consistent with sustained NO generation by NOS-2 rather than transient NO generation by constitutive NOS. Furthermore, it suggests that signalling is not through cGMP that is normally associated with rapid signalling in target cells. Previous reports have claimed that NO elevates cGMP in mast cells [34,35,53] but we have found that the effect of natural cell-derived NO on mast cell degranulation is not blocked by pharmacological inhibition of guanylyl cyclase (the enzyme that generates cGMP) and is not mimicked by 8-bromo-cGMP, a cell-permeant analogue of cGMP [47]. These results, in combination with the observed slow effect of NO, lead us to believe that NO does not act through cGMP to regulate mast cell degranulation, but more likely interacts chemically with protein targets to produce prolonged changes in responsiveness. This does not exclude the possibility that NO-induced cGMP may play roles in other aspects of mast cell behaviour.
Fig. 3.
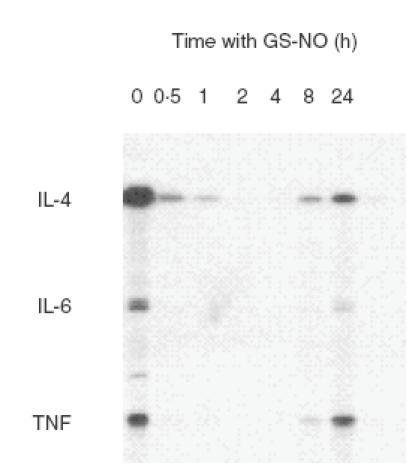
Effects of GS-NO on antigen-induced cytokine mRNA expression in RBL-2H3 mast cells. The cells were incubated with the NO donor for different time periods, challenged with antigen, and the RNA extracted 2 h later for analysis by RNase protection assay. Experiment performed by R.D. Koranteng.
NO exerts several effects on mast cells in addition to suppressing mediator production and release. For example, NO mediates the inhibitory effect of IFN-γ on adhesion of rat RBL-2H3 mast cells to fibronectin [51]; it inhibits generation of reactive oxygen species by rat peritoneal mast cells [54] and on these cells also up-regulates CD8 [55], the latter evidently through cGMP. Fibroblast-derived NO is cytostatic for cultured mouse mast cells [56] and NO is apoptotic towards a mouse mastocytoma cell line [57]. The effects of NO on apoptosis/cell survival is an active research area [58–60], and it would certainly be of interest if NO were to induce apoptosis of mast cells in vivo thereby influencing inflammation.
EFFECTS OF NITRIC OXIDE ON MAST CELL ACTIVATION AND MAST CELL-DEPENDENT INFLAMMATION IN VIVO
Evidence from animal studies supports an inhibitory action of NO on mast cell activation and mast cell-dependent inflammatory processes in vivo. Two groups have shown that treatment of rats with a NOS inhibitor induced mesenteric perivascular mast cell degranulation, and this was associated with enhanced mast cell-dependent leucocyte adhesion to the vascular endothelium and increased gut epithelial permeability [61,62]. Consistent with this, administration of a chemical NO donor to rats inhibited in vivo mast cell degranulation, and mast cell-dependent granulocyte endothelial adhesion and rolling, and microvascular leakage of albumin [63]. Further studies revealed that NO inhibits mast cell-dependent leucocyte adhesion by interacting with superoxide derived from the endothelium [64,65]. A recent study, again employing mast cell stabilizing drugs and a NOS inhibitor, showed that vascular permeability and associated pulmonary oedema in isolated rabbit lungs are inhibited by the suppressive actions of NO on mast cells [66]. In another model, inhibition of NOS enhanced allergen-induced histamine release by pig lungs in vivo[67]. In rats, injection of a protease-activated receptor-1 agonist into the hindpaw enhanced vascular permeability and induced paw oedema through a mast cell-dependent process. This response was enhanced by NOS inhibitors and suppressed by NO donors [68]. Overall, it appears from these studies, employing several in vivo models, that NO is an effective inhibitor of mast cell-dependent inflammatory events. The effects of NO on mast cell mediator release and mast cell dependent vascular changes are summarized in Fig. 4.
Fig. 4.
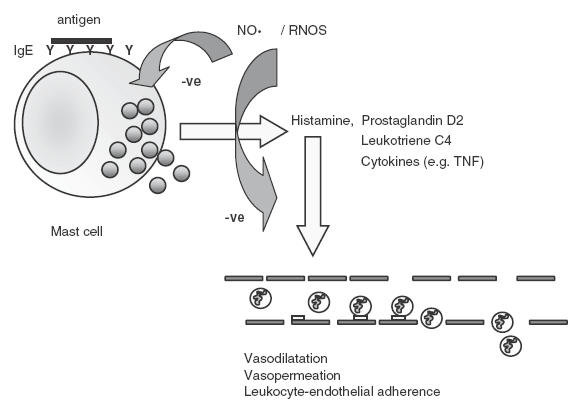
Inhibitory effects of NO on mast cell activation, mediator release and mast cell dependent vascular inflammatory events.
Nitric oxide in human asthma and animal models of asthma
NO is generated during human inflammatory diseases, the best documented example being asthma [12,69,70]. NO gas can readily be detected in the exhaled breath of asthmatics [71], and all three isoforms of NOS are present in the lung [69]. NO so generated may exert a combination of beneficial and harmful effects in relation to asthma [12,69,70]. For example, NOS-1 derived NO inhibits acetylcholine-mediated bronchoconstriction in human airways and NOS inhibitors increase bronchoconstrictor responses in animal models, suggesting a protective role for NOS-1 derived NO in asthma (reviewed in [69,70]). On the other hand, NO is a vasodilator and thus may promote plasma exudation and oedema, and facilitate cell recruitment to the tissue (pro-inflammatory effects).
The major cellular sources of high level NO production in the lung include NOS-2 expressing epithelial cells [72], macrophages, monocytes [12] and eosinophils [73]. A combination of cytokines (TNF, IL-1β and IFN-γ) induces NOS-2 expression in human primary epithelial cell cultures and epithelial cell lines [72] and this response is inhibited by glucocorticoids, IL-4 and IL-13 [25]. Primary cultured human epithelial cells from asthma patients generated less nitrite – a marker of NO production – than those from healthy subjects, and this was not related to drug therapy [74]. Human monocytes/macrophages are not so rich a source of NO as their rodent counterparts, and NO synthesis is activated by different stimuli, but nevertheless alveolar macrophages do represent a biologically significant source of human lung NO [12]. Human alveolar macrophages are also a target for regulation by NO – their production of inflammatory cytokines and associated activation of the transcription factor NFκB are inhibited by chemical NO donors [75,76]. Furthermore, levels of NFκB were reduced in bronchoalveolar lavage cells from asthmatic compared to healthy lungs, and this was related to increased NO production [75]. Because NFκB is a key component of the signalling pathway leading to expression of pro-inflammatory cytokines, these authors proposed that NO down-regulates cytokine production in the lung and is therefore protective in asthma [75,76]. Another recent study, which monitored the dynamics of generation of NO and its products in the lungs of atopic asthmatics challenged with allergen, suggested that NO exerts protective effects during the immediate asthmatic response by consuming reactive oxygen species [77]. Overall, such studies indicate an anti-inflammatory role for NO in human lungs.
Based on mouse gene knockout and in vitro T cell studies it has been claimed that NOS-2 derived NO may exacerbate asthma by inhibiting helper T lymphocyte (Th)1 and enhancing Th2 responses [18–20]. Although NO is certainly antiproliferative towards T lymphocytes, subsequent studies have failed to confirm a selective effect of NO on Th1 versus Th2 cells [13,78,79], casting doubt on the hypothesis of NO-driven asthma [20].
A study by Xiong et al.[80] revealed that genetic deletion of NOS-2 in mice led to a significant reduction in aeroallergen-induced appearance of eosinophils in bronchoalveolar lavage fluid (by up to 50%). Allergen-induced microvascular leakage, pulmonary oedema and airway occlusion were also less severe, while airway hyper-reactivity to methacholine (a defining feature of asthma) was unaltered compared to wild type mice [80]. De Sanctis et al.[81] confirmed that genetic deletion of NOS-2 in mice was without effect on allergen-induced airway hyper-responsiveness, but in contradiction to the Xiong study, genetic deletion of each of the three isoforms of NOS including NOS-2 had no effect on allergen-induced recruitment of eosinophils, granulocytes or mononuclear cells [81]. Deletion of NOS-1 or double deletion of NOS-1/NOS-3 reduced airway responsiveness, suggesting a contributory role of NOS-1 derived NO towards bronchial relaxation, but this was not linked any aspect of inflammation [81]. In wild type A/J mice, in which intranasal allergen challenge led to induction of NOS-2 dependent NO and recruitment of eosinophils to the lung, a selective NOS-2 inhibitor reduced eosinophil numbers in bronchoaleolar lavage fluid by approximately 60%[15]. Clearly then, there are conflicting messages from mouse studies of the role of NO in allergen-induced lung inflammation.
In allergen-sensitized pigs, NOS inhibition enhanced dramatically (by 16 times) allergen-induced lung histamine release into bronchoalveolar lavage fluid, and enhanced allergen-induced airway resistance [67]. The NOS inhibitor had no effect on histamine-induced airway resistance, thus demonstrating a protective effect of NO on pulmonary obstruction via inhibition of histamine release in vivo[67]. Likewise, in guinea pigs, NOS inhibitors revealed a protective effect of endogenous NO on airway reactivity [82]. Thus, in the pig and guinea pig, NO exerts beneficial effects on airway function.
CONCLUSIONS
A combination of in vitro and in vivo studies reveal inhibitory actions of NO on mast cell degranulation and mediator release, and mast cell-dependent inflammatory events including vasodilatation, vasopermeation and leucocyte-endothelial cell adhesion (Fig. 4). In inflammatory diseases, such as asthma, human and animal studies reveal possible pro-inflammatory as well as protective roles for NO, but overall the weight of evidence indicates a beneficial role for NO. Certainly the actions of NO in inflammation are complex and varied, not least because the radical targets multiple cell types including vascular smooth muscle cells, neurones and immune and inflammatory cells. Future research directions in inflammation and asthma are likely to cover the regulatory and signalling effects of NO on mast cells, T cells and other key cells, and the effects of NO donors and NOS inhibitors on disease progression.
Acknowledgments
The author thanks the Medical Research Council and The Wellcome Trust for recent support.
REFERENCES
- 1.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide. physiology, pathology and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 2.Lincoln J, Hoyle HVH, Burnstock G. Nitric Oxide in Health and Disease. Cambridge University Press. 1997 [Google Scholar]
- 3.Coleman JW. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001;1:1397–406. doi: 10.1016/s1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 4.Butler AR, Flitney FW, Williams DL. NO, nitrosonium ions, nitroxide ions, nitrosothiols and iron-nitrosyls in biology: a chemist's perspective. Trends Pharmacol Sci. 1995;16:18–22. doi: 10.1016/s0165-6147(00)88968-3. [DOI] [PubMed] [Google Scholar]
- 5.Wink DA, Hanbauer I, Grisham MB, et al. Chemical biology of nitric oxide: regulation and protective and toxic mechanisms. Curr Topics Cell Regulation. 1996;34:159–87. doi: 10.1016/s0070-2137(96)80006-9. [DOI] [PubMed] [Google Scholar]
- 6.Stamler JS. Redox signaling. nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–6. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 7.Kröncke K-D, Fehsel K, Suschek C, Kolb-Bachofen V. Inducible nitric oxide synthase-derived nitric oxide in gene regulation, cell death and cell survival. Int Immunopharmacol. 2001;1:1407–20. doi: 10.1016/s1567-5769(01)00087-x. [DOI] [PubMed] [Google Scholar]
- 8.Langrehr JM, Hoffman RA, Lancaster JR, Simmons RL. Nitric oxide – a new endogenous immunomodulator. Transplantation. 1993;55:1205–12. doi: 10.1097/00007890-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Bogdan C, Röllinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000;12:64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 10.Stuehr DJ, Marletta MA. Mammalian nitrate biosynthesis. mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci USA. 1985;82:7738–42. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuehr DJ, Marletta MA. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines or interferon-γ. J Immunol. 1987;39:518–25. [PubMed] [Google Scholar]
- 12.Thomassen MJ, Kavuru MS. Human alveolar macrophages and monocytes as a source and target for nitric oxide. Int Immunopharmacol. 2001;1:1479–90. doi: 10.1016/s1567-5769(01)00092-3. [DOI] [PubMed] [Google Scholar]
- 13.Van der Veen RC. Nitric oxide and T helper cell immunity. Int Immunopharmacol. 2001;1:1491–500. doi: 10.1016/s1567-5769(01)00093-5. [DOI] [PubMed] [Google Scholar]
- 14.Cifone MG, Ulisse S, Santoni A. Natural killer cells and nitric oxide. Int Immunopharmacol. 2001;1:1513–24. doi: 10.1016/s1567-5769(01)00095-9. [DOI] [PubMed] [Google Scholar]
- 15.Iijima H, Duguet A, Eum SY, Hamid Q, Eidelman DH. Nitric oxide and protein nitration are eosinophil dependent in allergen-challenged mice. Am J Respir Crit Care Med. 2001;163:1233–40. doi: 10.1164/ajrccm.163.5.2003145. [DOI] [PubMed] [Google Scholar]
- 16.Forsythe P, Gilchrist M, Kulka M, Befus AD. Mast cells and nitric oxide: control of production, mechanisms of response. Int Immunopharmacol. 2001;1:1525–41. doi: 10.1016/s1567-5769(01)00096-0. [DOI] [PubMed] [Google Scholar]
- 17.Bidri M, Féger F, Varadaradjalou S, Benhamouda N, Guillosson J-J, Arock M. Mast cells as a source and target for nitric oxide. Int Immunopharmacol. 2001;1:1543–58. doi: 10.1016/s1567-5769(01)00097-2. [DOI] [PubMed] [Google Scholar]
- 18.Taylor-Robinson AW, Liew FY, Severn A, Xu D, McSorley SJ, Garside P, Padron J, Phillips RS. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur J Immunol. 1994;24:980–4. doi: 10.1002/eji.1830240430. [DOI] [PubMed] [Google Scholar]
- 19.Wei X, Charles IG, Smith A, et al. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–11. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 20.Barnes PJ, Liew FY. Nitric oxide and asthmatic inflammation. Immunol Today. 1995;16:128–30. doi: 10.1016/0167-5699(95)80128-6. [DOI] [PubMed] [Google Scholar]
- 21.Thüring H, Stenger S, Gmehling D, Röllinghoff M, Bogdan C. Lack of inducible nitric oxide synthase activity in T cell clones and T lymphocytes from naive and Leishmania major-infected mice. Eur J Immunol. 1995;12:3229–34. doi: 10.1002/eji.1830251205. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong R. The physiological role and pharmacological potential of nitric oxide in neutrophil activation. Int Immunopharmacol. 2001;1:1501–12. doi: 10.1016/s1567-5769(01)00094-7. [DOI] [PubMed] [Google Scholar]
- 23.Nathan C, Xie Q-W. Nitric oxide synthases. roles, tolls and controls. Cell. 1994;78:915–8. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 24.Förstermann U, Pollock JS, Tracey WR, Nakane M. Isoforms of nitric oxide synthase: purification and regulation. Meth Enzymol. 1994;233:258–64. doi: 10.1016/s0076-6879(94)33029-8. [DOI] [PubMed] [Google Scholar]
- 25.Berkman N, Robichaud A, Robbins RA, Roesems G, Haddad EB, Barnes PJ, Chung KF. Inhibition of inducible nitric oxide synthase expression by interleulin-4 and interleukin-13 in human epithelial cells. Immunology. 1996;89:363–7. doi: 10.1046/j.1365-2567.1996.d01-745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–79. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 28.Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000;105:847–59. doi: 10.1067/mai.2000.106485. [DOI] [PubMed] [Google Scholar]
- 29.Drazen JM, Austen KF. Leukotrienes and airway responses. Am Rev Respir Dis. 1987;136:985–98. doi: 10.1164/ajrccm/136.4.985. [DOI] [PubMed] [Google Scholar]
- 30.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF/cachectin. Nature. 1990;19:274–6. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 31.Gordon JR, Galli SJ. Release of both preformed and newly synthesized tumor necrosis factor α (TNF/cachectin) by mouse mast cells stimulated via the FcɛRI. A mechanism for the sustained action of mast cell-derived TNF-α during IgE-dependent biological responses. J Exp Med. 1991;174:103–7. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galli SJ, Gordon JR, Wershil BK. Cytokine production by mast cells and basophils. Curr Opin Immunol. 1991;3:865–73. doi: 10.1016/s0952-7915(05)80005-6. [DOI] [PubMed] [Google Scholar]
- 33.Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-α. J Clin Invest. 1991;87:446–53. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salvemini D, Masini E, Pistelli A, Mannaioni PF, Vane J. Nitric oxide – a regulatory mediator of mast cell reactivity. J Cardiovasc Pharmacol. 1991;17:S258–64. [Google Scholar]
- 35.Masini E, Salvemini D, Pistelli A, Mannaioni PF, Vane JR. Rat mast cells synthesise a nitric oxide like factor which modulates the release of histamine. Agents Actions. 1991;33:61–3. doi: 10.1007/BF01993127. [DOI] [PubMed] [Google Scholar]
- 36.van Overveld FJ, Bult H, Vermeire PA, Herman AG. Nitroprusside, a nitrogen oxide generating drug, inhibits release of histamine and tryptase from human skin mast cells. Agents Actions. 1993;38:C237–8. [Google Scholar]
- 37.Iikura M, Takaishi T, Hirai K, Yamada H, Iida M, Koshino T, Morita Y. Exogenous nitric oxide regulates the degranulation of human basophils and rat peritoneal mast cells. Int Arch Allergy Immunol. 1998;115:129–36. doi: 10.1159/000023892. [DOI] [PubMed] [Google Scholar]
- 38.Masini E, Gambassi F, Di Bello MG, Mugnai L, Raspanti S, Mannaioni PF. Nitric oxide modulates cardiac and mast cell anaphylaxis. Agents Actions. 1994;41:C89–90. doi: 10.1007/BF02007780. [DOI] [PubMed] [Google Scholar]
- 39.Swieter M, Ghali WA, Rimmer C, Befus D. Interferon-α/β inhibits IgE-dependent histamine release from rat mast cells. Immunology. 1989;66:606–10. [PMC free article] [PubMed] [Google Scholar]
- 40.Coleman JW, Buckley MG, Holliday MR, Morris AG. Interferon-γ inhibits serotonin release from mouse peritoneal mast cells. Eur J Immunol. 1991;21:2559–64. doi: 10.1002/eji.1830211037. [DOI] [PubMed] [Google Scholar]
- 41.Holliday MR, Banks EM, Dearman RJ, Kimber I, Coleman JW. Interactions of IFN-γ with IL-3 and IL-4 in the regulation of serotonin and arachidonate release from mouse peritoneal mast cells. Immunology. 1994;82:70–4. [PMC free article] [PubMed] [Google Scholar]
- 42.Coleman JW, Holliday MR, Kimber I, Zsebo KM, Galli SJ. Regulation of mouse peritoneal mast cell secretory function by stem cell factor, IL-3 or IL-4. J Immunol. 1993;150:556–62. [PubMed] [Google Scholar]
- 43.Buckley MG, Williams CM, Thompson J, Pryor P, Ray K, Butterfield JH, Coleman JW. IL-4 enhances IL-3 and IL-8 gene expression in a human leukemic mast cell line. Immunology. 1995;84:410–5. [PMC free article] [PubMed] [Google Scholar]
- 44.Coleman JW. Regulation of mast cell secretion by interferon-γ and nitric oxide. In: Marone G, Lichtenstein LM, Galli SJ, editors. Mast cells and Basophils. London: Academic Press; 2000. pp. 221–32. [Google Scholar]
- 45.Eastmond NC, Banks EMS, Coleman JW. Nitric oxide inhibits IgE-mediated degranulation of mast cells and is the principal intermediate in IFN-γ-induced suppression of exocytosis. J Immunol. 1997;159:1444–50. [PubMed] [Google Scholar]
- 46.Brooks B, Briggs DM, Eastmond NC, Fernig DG, Coleman JW. Presentation of IFN-γ to nitric oxide-producing cells: a novel function for mast cells. J Immunol. 2000;164:573–9. doi: 10.4049/jimmunol.164.2.573. [DOI] [PubMed] [Google Scholar]
- 47.deSchoolmeester ML, Eastmond NC, Dearman RJ, Kimber I, Basketter DA, Coleman JW. Reciprocal effects of interleukin-4 and interferon-γ on immunoglobulin E-mediated mast cell degranulation: a role for nitric oxide but not peroxynitrite or cyclic guanosine monophosphate. Immunology. 1999;96:138–44. doi: 10.1046/j.1365-2567.1999.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lau HY, Chow SM. Nitric oxide does not affect histamine release from rat peritoneal mast cells. Inflamm Res Supplement. 1999;1:S27–8. doi: 10.1007/s000110050382. [DOI] [PubMed] [Google Scholar]
- 49.Peh KH, Moulson A, Wan BY, Assem EK, Pearce FL. Role of nitric oxide in histamine release from human basophils and rat peritoneal mast cells. Eur J Pharmacol. 2001;425:229–38. doi: 10.1016/s0014-2999(01)01205-5. [DOI] [PubMed] [Google Scholar]
- 50.Bidri M, Bidri M, Ktorza S, Vouldoukis I, Le Goff L, Debre P, Guillosson JJ, Arock M. Nitric oxide pathway is induced by Fc epsilon RI and up-regulated by stem cell factor in mouse mast cells. Eur J Immunol. 1997;27:2907–13. doi: 10.1002/eji.1830271124. [DOI] [PubMed] [Google Scholar]
- 51.Wills FL, Gilchrist M, Befus AD. Interferon-γ regulates the interaction of RBL-2H3 cells with fibronectin through production of nitric oxide. Immunology. 1999;97:481–9. doi: 10.1046/j.1365-2567.1999.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilchrist M, Savoie M, Nohara O, Wills FL, Wallace JL, Befus AD. Nitric oxide synthase and nitric oxide production in in vivo– derived mast cells. J Leukoc Biol. 2002;71:618–24. [PubMed] [Google Scholar]
- 53.Bidri M, Becherel PA, Le Goff L, Pieroni L, Guillosson JJ, Debre P, Arock M. Involvement of cyclic nucleotides in the immunomodulatory effects of nitric oxide on murine mast cells. Biochem Biophys Res Commun. 1995;210:507–17. doi: 10.1006/bbrc.1995.1689. [DOI] [PubMed] [Google Scholar]
- 54.Brooks AC, Whelan CJ, Purcell WM. Reactive oxygen species generation and histamine release by activated mast cells: modulation by nitric oxide synthase inhibition. Br J Pharmacol. 1999;128:585–90. doi: 10.1038/sj.bjp.0702838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nohara O, Kulka M, Dery RE, Wills FL, Hirji NS, Gilchrist M, Befus AD. Regulation of CD8 expression in mast cells by exogenous or endogenous nitric oxide. J Immunol. 2001;167:5935–9. doi: 10.4049/jimmunol.167.10.5935. [DOI] [PubMed] [Google Scholar]
- 56.Kim HM. Nitric oxide released from Swiss 3T3 fibroblasts acts as a cytostatic agent for cultured mast cells. Pharmacology. 1997;55:285–91. doi: 10.1159/000139540. [DOI] [PubMed] [Google Scholar]
- 57.Kitajima I, Kawahara K, Nakajima T, Soejima Y, Matsuyama T, Maruyama I. Nitric oxide-mediated apoptosis in murine mastocytoma. Biochem Biophys Res Commun. 1994;204:244–51. doi: 10.1006/bbrc.1994.2451. [DOI] [PubMed] [Google Scholar]
- 58.Kim PKM, Zamora R, Petrosko P, Billiar TR. The regulatory role of nitric oxide in apoptosis. Int Immunopharmacol. 2001;1:1421–41. doi: 10.1016/s1567-5769(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 59.Kröncke KD, Brenner HH, Rodriguez ML, Etzkorn K, Noack EA, Kolb H, Kolb-Bachofen V. Pancreatic islet cells are highly susceptible towards the cytotoxic effects of chemically generated nitric oxide. Biochim Biophys Acta. 1993;1182:221–9. doi: 10.1016/0925-4439(93)90144-p. [DOI] [PubMed] [Google Scholar]
- 60.Fehsel K, Kröncke KD, Meyer KL, Huber H, Wahn V, Bachofen V. Nitric oxide induces apoptosis in mouse thymocytes. J Immunol. 1995;155:2858–65. [PubMed] [Google Scholar]
- 61.Kanwar S, Wallace JL, Befus D, Kubes P. Nitric oxide synthesis inhibition increases epithelial permeability via mast cells. Am J Physiol. 1994;266:G222–9. doi: 10.1152/ajpgi.1994.266.2.G222. [DOI] [PubMed] [Google Scholar]
- 62.Kimura M, Mitani H, Bandoh T, Totsuka T, Hayashi S. Mast cell degranulation in rat mesenteric venule. effects of L-NAME, methylene blue, and ketotifen. Pharmacol Res. 1999;39:397–402. doi: 10.1006/phrs.1999.0451. [DOI] [PubMed] [Google Scholar]
- 63.Gaboury JP, Niu XF, Kubes P. Nitric oxide inhibits numerous features of mast cell-induced inflammation. Circulation. 1996;15:318–26. doi: 10.1161/01.cir.93.2.318. [DOI] [PubMed] [Google Scholar]
- 64.Kubes P, Kanwar S, Niu XF, Gaboury JP. Nitric oxide synthesis inhibition induces leukocyte adhesion via superoxide and mast cells. FASEB J. 1993;7:1293–9. doi: 10.1096/fasebj.7.13.8405815. [DOI] [PubMed] [Google Scholar]
- 65.Niu XF, Ibbotson G, Kubes P. A balance between nitric oxide and oxidants regulates mast cell-dependent neutrophil–endothelial cell interactions. Circ Res. 1996;79:992–9. doi: 10.1161/01.res.79.5.992. [DOI] [PubMed] [Google Scholar]
- 66.Mundy AL, Dorrington KL. Inhibition of nitric oxide synthesis augments pulmonary oedema in isolated perfused rabbit lung. Br J Anaesth. 2000;85:570–6. doi: 10.1093/bja/85.4.570. [DOI] [PubMed] [Google Scholar]
- 67.Middelveld RJ, Zetterquist WC, Bergman D, Alving K. Nitric oxide synthase inhibition augments acute allergic reactions in the pig airways in vivo. Eur Respir J. 2000;16:836–44. doi: 10.1183/09031936.00.16583600. [DOI] [PubMed] [Google Scholar]
- 68.Kawabata A, Kuroda R, Nishikawa H, Asai T, Kataoka K, Taneda M. Enhancement of vascular permeability by specific activation of protease-activated receptor-1 in rat hindpaw: a protective role of endogenous and exogenous nitric oxide. Br J Pharmacol. 1999;126:1856–62. doi: 10.1038/sj.bjp.0702513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barnes PJ. NO or no NO in asthma? Thorax. 1996;51:218–20. doi: 10.1136/thx.51.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kharatinov SA, Barnes PJ. Clinical aspects of exhaled nitric oxide. Eur Respir J. 2000;16:781–92. doi: 10.1183/09031936.00.16478100. [DOI] [PubMed] [Google Scholar]
- 71.Kharitonov SA, Yates D, Robbins RA, Logan-Sinclair R, Shinebourne EA, Barnes PJ. Increased nitric oxide in exhaled air of asthmatics. Lancet. 1994;343:133–5. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- 72.Robbins RA, Barnes PJ, Springall DR, et al. Expression of inducible nitric oxide in human lung epithelial cells. Biochem Biophys Res Commun. 1994;203:209–18. doi: 10.1006/bbrc.1994.2169. [DOI] [PubMed] [Google Scholar]
- 73.MacPherson JC, Comhair SA, Erzurum SC, Klein DF, Lipscomb MF, Kavuru MS, Samoszuk MK, Hazen SL. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol. 2001;166:5763–72. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- 74.Donnelly LE, Barnes PJ. Expression and regulation of inducible nitric oxide synthase from human primary airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26:144–51. doi: 10.1165/ajrcmb.26.1.4477. [DOI] [PubMed] [Google Scholar]
- 75.Thomassen MJ, Raychaudhuri B, Dweik RA, et al. Nitric oxide regulation of asthmatic airway inflammation with segmental allergen challenge. J Allergy Clin Immunol. 1999;104:1174–82. doi: 10.1016/s0091-6749(99)70010-2. [DOI] [PubMed] [Google Scholar]
- 76.Raychaudhuri B, Dweik R, Connors MJ, et al. Nitric oxide blocks nuclear factor-kappaB activation in alveolar macrophages. Am J Respir Cell Mol Biol. 1999;21:311–6. doi: 10.1165/ajrcmb.21.3.3611. [DOI] [PubMed] [Google Scholar]
- 77.Dweik RA, Comhair SA, Gaston B, et al. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci USA. 2001;98:2622–7. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Veen RC, Dietlin TA, Pen L, Gray JD. Nitric oxide inhibits the proliferation of T-helper 1 and 2 lymphocytes without reduction in cytokine secretion. Cell Immunol. 1999;193:194–201. doi: 10.1006/cimm.1999.1471. [DOI] [PubMed] [Google Scholar]
- 79.Bauer H, Jung T, Tsikas T, Stichtenoth DO, Frolich JC, Neumann C. Nitric oxide inhibits the secretion of T-helper 1 and T-helper 2-associated cytokines in activated. T cells. Immunology. 1997;90:205–11. doi: 10.1046/j.1365-2567.1997.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiong Y, Karupiah G, Hogan SP, Foster PS, Ramsay AJ. Inhibition of allergic airway inflammation in mice lacking nitric oxide synthase 2. J Immunol. 1999;162:445–52. [PubMed] [Google Scholar]
- 81.De Sanctis GT, MacLean JA, Hamada K, et al. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Medical. 1999;189:1621–9. doi: 10.1084/jem.189.10.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schuiling M, Zuidhof AB, Bonouvrie MA, Venema N, Zaagsma J, Meurs H. Role of nitric oxide in the development and partial reversal of allergen-induced airway hyperreactivity in conscious, unrestrained guinea-pigs. Br J Pharmacol. 1998;123:1450–6. doi: 10.1038/sj.bjp.0701738. [DOI] [PMC free article] [PubMed] [Google Scholar]


