Abstract
We investigated the effect of N-acetylcysteine (NAC) on normal human B cell functions. We found that NAC significantly inhibited both the induction of the specific antibody response to the T-dependent antigen Candida albicans and T-dependent pokeweed mitogen (PWM)-induced polyclonal Ig production. NAC did not induce either cell death due to a non-specific toxicity or apoptosis. The NAC-induced inhibitory effect might be a functional consequence of: (i) a down-regulation of the expression on the B cell surface of CD40 and CD27 co-stimulatory molecules and (ii) a down-regulation of interleukin (IL-4) production. In contrast, NAC up-regulated interferon-γ (IFN-γ) production. NAC did not induce any effect on the T cell-independent B cell polyclonal activation system. These results indicate that NAC down-regulates T dependent B cell activation and leads to T helper cell type 1 (Th1) polarization.
Keywords: antigen-specific antibody response, co-stimulatory molecules, human B cells, N-acetylcysteine, polyclonal immunoglobulin production
INTRODUCTION
NAC is an antioxidant drug that enters cells readily and interferes with or counteracts reactive oxygen species-induced subcellular changes by different mechanisms. NAC acts both as a scavenger for reactive oxidative intermediates and as a precursor for glutathione (GSH), the co-substrate for the radical eliminating enzyme GSH peroxidase [1]. NAC is used classically as a mucolytic agent and, in view of its ability to block human immunodeficiency virus (HIV)-1 replication [2], and to restore the intracellular levels of GSH in HIV-infected patients [3], is used as a therapeutic agent in asymptomatic HIV-infected patients and in AIDS treatment trials [4].
Several in vitro studies indicated that NAC exerts immunomodulatory activity. Indeed, NAC activates T lymphocytes by increasing resistance to oxidative stress-mediated apoptosis [5], cytotoxic properties [6], synthesis and turnover of IL-2 receptors, IL-2 production and proliferation [7]. In a recent study, Bengtsson et al. [8], demonstrated that NAC decreases cytokine levels and down-regulates the expression of CD30 on human allergen-specific Th0 and Th2 cells. Our previous studies have demonstrated that NAC up-regulates natural killer cell-mediated cytotoxic activity by a mechanism involving cytoskeleton function [9]. Verhaselt et al. demonstrated that NAC impairs the generation of primary human T cell responses in humans through its inhibitory action on dendritic cells [10].
Few studies have been performed regarding the effect of NAC on humoral immunity. In this context, Jeannin et al. demonstrated that NAC inhibits IL-4 production in human T cells and reduces the production of IgE and IgG4 by B cells [11]. In studies using B cell lines, Yanagihara et al. [12] showed that NAC regulates IgE isotype switching by inhibiting the activation of NF-κB. However, no evidence is available about the effect of NAC on the induction and regulation of the antigen specific antibody response.
In the present study we evaluated the effect of NAC in an in vitro system whereby lymphocytes isolated from normal human blood are induced by the T cell-dependent antigen Candida albicans to mount a specific antibody response. We found that NAC induced a significant down-regulation of the specific antibody response by a mechanism involving an early down-regulation on the B cell surface of CD40 and CD27 co-stimulatory molecules and a significant down-regulation of IL-4 production accompanied by a significant up-regulation of IFN-γ production.
MATERIALS AND METHODS
Reagents
NAC was purchased from the Zambon Group (Italy). The drug was dissolved in RPMI 1640 and the pH was adjusted to 7·4 by the addition of NaHCO. C. albicans (strain BP, serotype A) cells and mannoprotein purified from C. albicans cell wall were prepared as described previously [13,14]. Pokeweed mitogen (PWM) was purchased from Gibco (Paisley, UK) and was used at a final dilution of 1:100. Staphylococcus aureus Cowan Strain (SAC) was purchased from Calbiochem (La Jolla, CA, USA) and used at final concentration of 1:10·000 (v/v). Recombinant human IL-2 was purchased from Janssen Biochimica (Gell, Belgium) and used at a final concentration of 50 units/ml. Anti-CD27 (R&D Systems, Minneapolis, USA) and anti-CD40 (PharMingen, San Diego, CA, USA) monoclonal antibodies (MoAbs) were used at a concentration of 1 μg/ml. Flat-bottomed microtitre plates (96-well) were coated with recombinant human CD40 ligand (CD40L) (Alexis, Grunberg, Germany) at a concentration of 3 μg/ml and incubated for 1h at 37°C. The irrelevant anti-2,4,6-trinitrophenyl (TNP) antibody was purchased from Intracel (London, UK) and used at a concentration of 1 μg/ml.
Cell preparation
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coats obtained from healthy volunteer blood donors by the Italian Red Cross Transfusion Center, Rome, Italy. PBMC were washed and suspended in the appropriate tissue culture medium and stimulated with PWM.
B cells were purified from PBMC by positive selection using magnetic cell separation columns and CD19 Microbeads (Milteny Biotec GmbH, Bergisch Gladbach, Germany). The highly enriched B cells (>90% CD19+) were suspended in the appropriate tissue culture medium and stimulated with SAC + IL-2.
When PBMC were to be used in cultures aimed at inducing specific antibody responses, heparinized blood was first passed through a synthetic wool (Aldrich, Milan, Italy) column and then layered over Ficoll. This procedure allows the removal of strongly adhering suppressor cells, as described previously in detail [15]. Cells were washed and suspended in the appropriate tissue culture medium.
Induction of an antigen-specific antibody response
The method described by Giacomini et al. [16] was followed. Briefly, PBMC were suspended at a concentration of 7 × 106/ml in Iscove's modified Dulbecco's medium (IMDM, Sigma Chemical Co., St Louis, MO, USA) supplemented with 50 U/ml of both penicillin and streptomycin, 4% polyethylene glycol (PEG, Serva, Heidelberg, Germany, MW 6000), 2% heat-inactivated and C. albicans absorbed human AB serum, 8% fetal calf serum (FCS). Antigen was added in the form of 0·05 ml of 1·5 × 108 C. albicans cells/ml in HBSS per ml of cell suspension. Five replicate cultures per condition were established in the wells of Microtest plates (Falcon Plastics, Lincoln Park, NJ, USA) at a concentration of 0·7 × 106 cells per well. Cultures were incubated at 37°C in an humidified 5% CO2 incubator. At days 9–10 cultures were harvested, the five replicate wells were pooled and assayed for either specific antibody secreting cells (ASC) or for IL-4-secreting cells (IL-4-SC) or IFN-γ secreting cells (IFN-γ SC) by the ELISPOT assay. ASC, IL-4-SC and IFN-γ-SC were expressed per number of recovered cells at the end of the culture period. Viable cell counts were performed on the harvested samples by ethidium bromide/acridine orange staining.
PWM or SAC + IL-2 induction of Ig production
PBMC or purified B cells were suspended at 1 × 106/ml in RPMI, supplemented with 10% heat-inactivated FCS, l-glutamine and antibiotics and stimulated with PWM or SAC + IL-2, respectively. The cell suspensions were distributed in aliquots of 0·1 ml in the wells of Microtest plates. The cultures were incubated at 37°C in a humidified 5% CO2 incubator. After 7 days, 10 wells were pooled and cells were assayed for the percentage of intracellular Ig positive-cells by flow cytometric analysis.
ELISPOT assay for antibody-secreting cells
Cells secreting specific anti- C. albicans antibody were enumerated by a modification of the ELISPOT test [17]. Flat bottomed microtitre plates (96-well) (Dynatech M129A, Alexandria, VA, USA) were coated with 0·1 ml/well of 10 μg/ml mannoprotein purified from C. albicans in carbonate-bicarbonate buffer, pH 9·7, overnight at 4°C. The plates were washed twice with phosphate buffered saline (PBS)-0·05% Tween 20 (Sigma Chemical Co.) and incubated for 1h at 37°C with PBS containing 3% gelatin (Sigma Chemical Co.) as a blocking agent. The plates were rinsed, the cells were then added and incubated at 37°C in a 5% CO2 incubator for 3 h. The wells were subsequently washed and then incubated overnight at 4°C with 100 μl/well of an optimal dilution of alkaline phosphatase (AP) conjugated goat antihuman immunoglobulin (Ig) G (Sigma Chemical Co.) preparation. After extensive washing with PBS-Tween, 100 μl of the AP substrate 5-bromo-4-chloro-3-indolylphosphate (BCIP, Sigma Chemical Co., 1 mg/ml) in 1m 2-amino-2 methyl-1-propanol buffer (AMP, Sigma Chemical Co.) containing 5 mm MgCl2, 0·01% Triton X-405 (Sigma Chemical Co.) and 0·01% NaN3 were added to each well. The plates were incubated for 1h at room temperature. The supernatants were then discarded and the wells rinsed with deionized water. The plates were allowed to dry and the spots were enumerated under a stereomicroscope with 40-fold magnification.
Detection of the percentage of B cells producing intracellular Ig by flow cytometry
Analysis of the percentage of B cells producing intracellular Ig was performed either on PBMC cultures stimulated with PWM or on purified B cells stimulated with SAC + IL-2 and either treated with NAC for 7 days at 37°C or left untreated. After incubation the cells were washed with calcium-magnesium free HBSS (Hyclone, UK) and fixed with 0·5 ml of ice-cold 4% paraformaldehyde for 10 min at 4°C. Cells were permeabilized with 1 ml HBSS/0·1% saponin (Sigma Chemical Co.) for 10 min and incubated with FITC-labelled goat antihuman total Ig (Cappel) or with FITC-conjugated antimouse total Ig (Cappel), as a negative control, for 30 min at room temperature. Cells were washed and, for PWM-stimulated PBMC, B cell surface phenotype determination was assayed by incubating cells with a PE-conjugated antibody to CD20 (PharMingen) for 30 min at 4°C and finally resuspending the cells in HBSS. Samples were then analysed using a FACScan flow cytometer and Cell Quest software (Becton-Dickinson).
Assay for interleukin-4 (IL-4) IFN-γ-secreting cells
The ELISPOT test, used to enumerate cells secreting IFN-γ or IL-4, was based essentially on the method described by Versteegeg et al. [18], but carried out with some modifications. Briefly, flat-bottomed microtitre plates (96-well) (Nunc-Immunoplate Maxisorp, Roskilde, Denmark) were coated with 0·1 ml/well mouse antihuman IFN-γ MoAb or mouse antihuman IL-4 MoAb (Genzyme, Cambridge, MA, USA) 10 μg/ml in carbonate-bicarbonate buffer pH 9·7, overnight at 4°C. Plates were washed with PBS −0·05% Tween20 (Sigma Chemical Co.) and then blocked with 3% gelatin (Sigma Chemical Co.) in PBS. After exhaustive washings, the cultured cells were added to the wells and incubated overnight at 37°C in a 5% CO2 incubator. After removal of the cells, the wells were incubated for 90 min at 37°C with an optimal dilution of rabbit antihuman IFN-γ or IL-4 (Genzyme), then washed and incubated for 90 min at 37°C with an optimal dilution of alkaline phosphatase (AP)-conjugated goat antirabbit IgG (Sigma Chemical Co.). After extensive washing, 0·1 ml of the AP substrate BCIP (1 mg/ml) in 1 ml l2-amino-2-methyl-1-propanol buffer was added. After 1h incubation at room temperature the plates were rinsed with deionized water and allowed to dry. The blue spots were counted under a stereomicroscope with × 40 magnification.
Apoptosis detection
Analysis of B cell apoptosis was performed using PBMC cultures stimulated with C. albicans antigen in the presence or absence of NAC at concentrations of 5 mm, 10 mm and 20 mm. We used 10 mm 2-deoxy-d-ribose (Sigma Chemical Co.) as a positive control for B cell apoptosis [19]. After incubation for 18 h, cells were washed with PBS and incubated with PE-conjugated antihuman CD20 for 30 min at 4°C. After further washing, the cells were resuspended in binding buffer before the addition of 5 μl of FITC–conjugated Annexin V (PharMingen). The percentage of B cells in early apoptosis (PE-conjugated CD20 and FITC-conjugated AnnexinV-positive cells) were then quantified by FACS.
Flow cytometric analysis
Analysis of the percentage of double positive CD20/CD40 or CD20/CD27 cells was performed on PBMC cultures stimulated with antigen or PWM in the presence or absence of NAC (20 mm, 10 mm and 5 mm). After 20, 40 and 72h of incubation, the cells were washed with PBS containing 2% FCS and 0·01 NaN3 and then double-stained with PE-conjugated antibody to CD20 and FITC-conjugated antibody to either CD40 (PharMingen) or CD27 (PharMingen). As a negative control, cells were stained with matched control Ig isotypes (Becton Dickinson). Cells were analysed in a FACScan equipped with Cell Quest software (Becton Dickinson).
Intracellular CD40 and CD27 staining was performed on C. albicans stimulated PBMC cultured for 24, 48 and 72h at 37°C. Cells were washed with calcium–magnesium-free Hanks's balanced salt solution (HBSS; Hyclone) and fixed with 0·5 ml of ice-cold 4% paraformaldehyde for 10 min at 4°C. Cells were permeabilized with 1 ml HBSS/0·1% saponin (Sigma Chemicals) for 10 min and incubated with anti-CD20 PE and with anti-CD40 FITC or anti-CD27 FITC for 45 min at room temperature. The cells were washed, resuspended in PBS and analysed using a flow cytometer (Becton-Dickinson FACScan).
Statistical analysis
Values are presented as mean ± standard error of the mean (s.e.m.). The statistical significance of the differences was calculated using a paired Student's t-test. A P-value of <0·05 was considered to be statistically significant.
RESULTS
NAC inhibits the induction of specific antibody-secreting cells
We first investigated whether NAC exerted any effect on the induction of ASC to C. albicans antigen in cultures of human PBMC. Cells were seeded in co-culture with C. albicans, in a culture system allowing the induction of a specific antibody response to this antigen [15]. This response has been shown previously to be secondary, peaking at days 7–10 of culture [20]. For this, reason all experiments were assayed in this time-frame and the specific antibody response evaluated by enumerating specific ASC of the IgG class only. Increasing concentrations of NAC (from 0·05 to 20 mm) were added at the beginning of C. albicans-stimulated culture. As shown in Fig. 1a, NAC significantly decreased the number of anti- C. albicans IgG ASC in a dose-dependent manner reaching inhibition of 70% at a concentration of 5 mm (P <0·05) and up to 95% at a concentration of 10 and 20 mm (P <0·05). Inhibition of specific IgG ASC cannot be ascribed to a toxic effect of NAC on cell cultures. In fact, viability tests performed at the time of culture harvesting, showed that even in cultures where the anti- C. albicans response was inhibited up to 95%, the number of viable cells recovered could be equal to or higher than in control untreated cultures (data not shown).
Fig. 1.
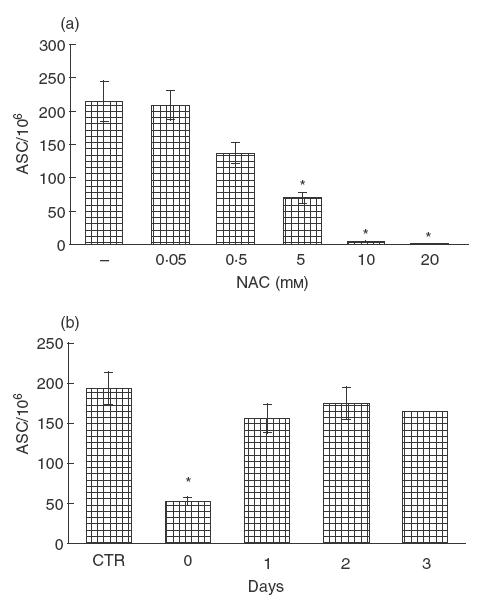
Effect of NAC on the induction of the specific antibody response to C. albicans. (a) Increasing amounts of NAC were added to C. albicans-stimulated cultures; after 9–10 days, cells were assayed for anti- Candida antibody-secreting cells (ASC). Data represent mean ± s.e.m. of six different experiments. * P <0·05. (b) The antigen-stimulated cultures were treated with or without NAC (5 mm) at the beginning of culture or after 1, 2 and 3 days. At day 9 cells were assayed for anti- Candida ASC. The data are expressed as mean ± s.e.m. from six independent experiments. * P <0·05 versus control.
We then performed a time-course for NAC-induced inhibition of the specific antibody response. The results shown in Fig. 1b indicate that NAC added at beginning of culture induced significant inhibition (P <0·05), while no inhibitory effect was evident at late time points (1, 2, 3 days).
NAC does not affect B cell viability
To evaluate whether NAC-induced inhibition of specific antibody production was due to its cytopathic effect on B cells, we evaluated B cell viability by performing double-staining FACS analysis using Annexin V-FITC and anti CD20-PE. As illustrated in Table 1, after 18h of culture NAC, at all concentrations used, the percentage of double-positive cells was not modified. Similar results were obtained at 40h of culture (data not shown). Using 2-deoxy-d-ribose as a positive control, we found 30% of cells were Annexin V+/CD20+.
Table 1.
Effect of NAC on B cells viability in antigen-stimulated cultures
| Cell labelling Annexin V+/CD20+ | |
|---|---|
| CTR | 5·3 |
| 2-Deoxy-d-ribose | 30 |
| NAC 5 mm | 5·5 |
| NAC 10 mm | 5 |
| NAC 20 mm | 4·9 |
After 18h of cultures the percentage of B cells double-positive in early apoptosis was analysed by FACS. One representative experiment of three is shown.
NAC down-regulates membrane expression of co-stimulatory molecules on C. albicans-stimulated B cells
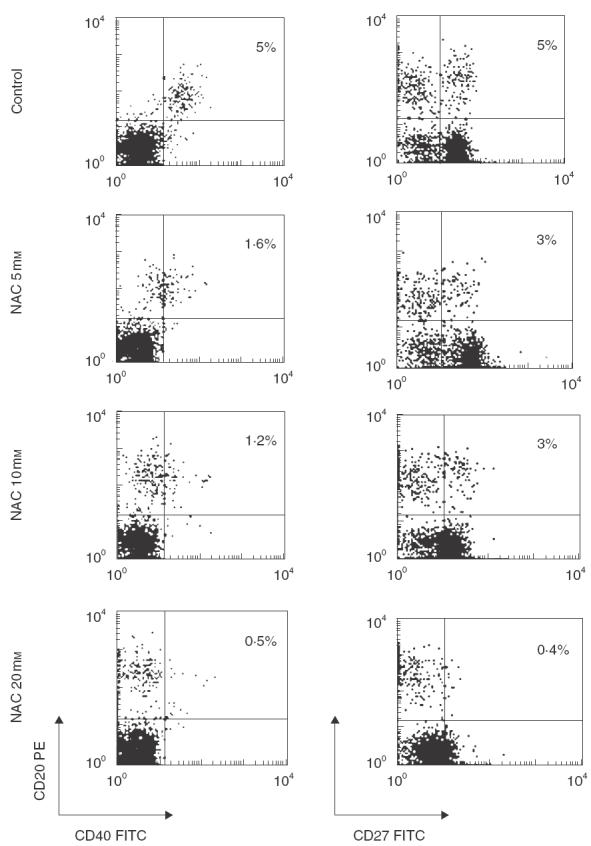
Because B cell activation requires co-stimulatory signals provided by direct specific contact with helper T lymphocytes, we analysed the effect of NAC on the expression of such co-stimulatory molecules on the surface of B cells in C. albicans-stimulated cultures. We found that NAC induced an early (18 h) dose-dependent down-regulation of the surface expression of both CD40 and CD27 molecules (Fig. 2) on C. albicans-stimulated CD20+ cells. Similar results were obtained after 48 and 72h of culture (data not shown). Moreover, NAC induced an early (18 h) down-regulation of surface expression of both CD40 and CD27 molecules on non-activated B cells (Fig. 3).
Fig. 2.
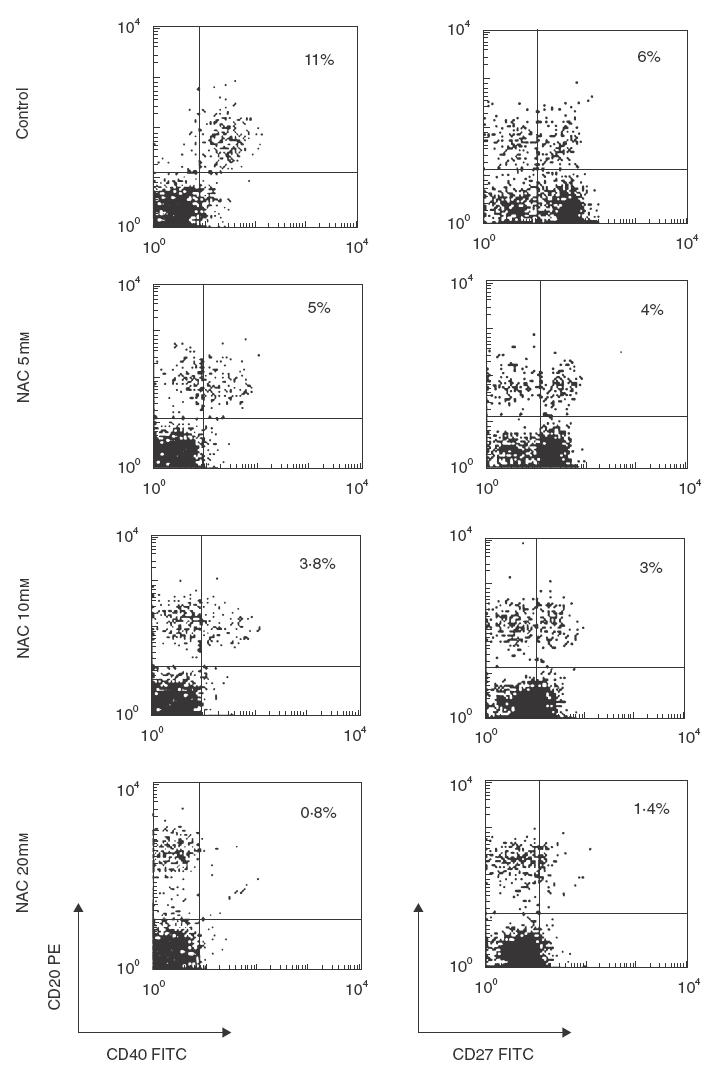
Effect of NAC on the surface expression of co-stimulatory molecules on antigen-activated B cells. PBMC were cultured with C. albicans in the absence or presence of increasing amounts of NAC. After 18h cells were harvested and two-colour analysis was carried out staining with FITC-CD40 and PE-CD20 (left panel) or FITC-CD27 and PE-CD20 (right panel). Data are shown as density plots with green (FITC) fluorescence for CD40 and CD27 on the x-axis and orange (PE) fluorescence for CD20 on the y-axis in logarithmic scale. The percentage of double-positive cells is indicated. The data are from one representative experiment of six performed.
Fig. 3.
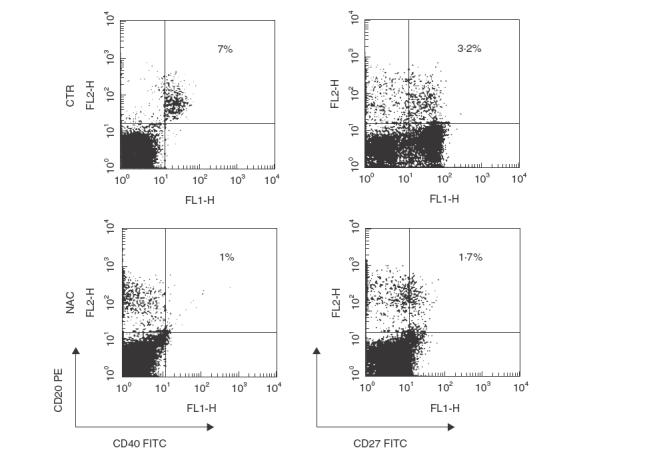
Effect of NAC on the surface expression of co-stimulatory molecules on non-activated B cells. PBMC were cultured in the absence or presence of 20 mm NAC. After 18h cells were harvested and two-colour analysis was carried out staining with FITC-CD40 and PE-CD20 (left panel) or FITC-CD27 and PE-CD20 (right panel). Data are shown as density plots with green (FITC) fluorescence for CD40 and CD27 on the x-axis and orange (PE) fluorescence for CD20 on the y-axis in logarithmic scale. The percentage of double-positive cells is indicated. The data are from one representative experiment of two performed.
It should be pointed out that NAC also down-regulated the expression of CD40 and CD27 molecules on CD20− cells (Fig. 2). In particular, we found that the expression of CD27 was reduced to less than 86% on the surface of CD4+ (CD20−) cells (data not shown) while the expression of CD40 was reduced to less than 85% on the surface of CD14+ (CD20−) (data not shown). Moreover, we found that the expression of CD70 (CD27 ligand) was reduced to less than 45% by NAC (data not shown).
In order to define the mechanism of NAC-mediated down-regulation of CD27 and CD40 expression, we performed experiments utilizing saponin-permeabilized cells. We found that in NAC-treated C. albicans-stimulated cultures CD27 and CD40 molecules were down-regulated by distinct mechanisms. In fact, after 24 and 48h CD27 expression in saponin-permeabilized cells was not modified by NAC, suggesting internalization of CD27. Expression was reduced after 72 h, however, due most probably to the processing of the internalized molecules. In contrast, CD40 expression in saponin-permeabilized cells was reduced by NAC after only 24 h, suggesting release of CD40 into the milieu (Fig. 4).
Fig. 4.
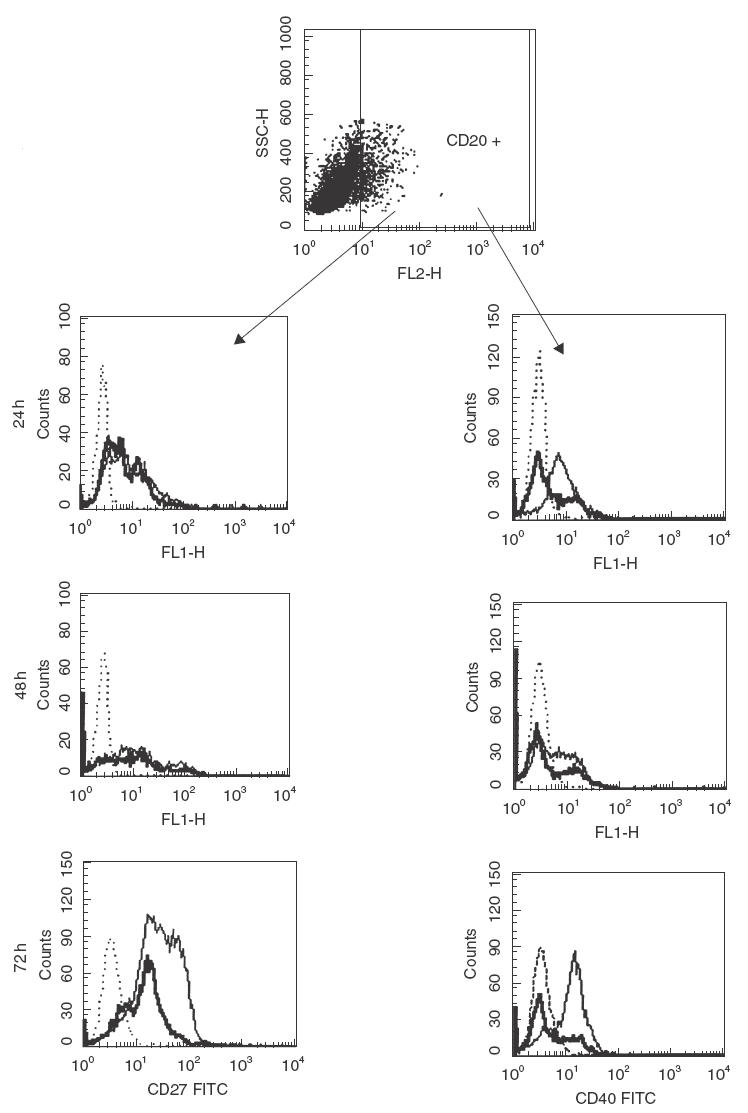
Effect of 20 mm NAC on CD40 and CD27 intracellular expression in C. albicans-activated B cells. CD20 lineage marker cells (B cells) were defined by SSC versus CD20 PE dot plot. Then, CD20+ cells were gated electronically and analysed for expression of CD27 FITC or CD40 FITC. Expression of CD40 or CD27 was presented by single-colour histograms of log10 fluorescence intensity obtained with an isotype-matched control (dotted histogram) and specific CD40 MoAb (left) or CD27 MoAb (right) for control (thin histogram) or NAC-treated cells (solid histogram). The data are from one representative experiment of two performed.
Anti-human CD40 and CD27 antibodies inhibit the induction of an anti- C. albicans-specific antibody response
To verify whether the observed decrease in co-stimulatory molecule expression on the B cell surface was responsible for the NAC inhibitory effect on the induction of a specific antibody response, anti-CD27 or anti-CD40 MoAbs were added at the start of culture. Figure 5 shows that anti-CD40 and anti-CD27 MoAbs induced a level of inhibition of the anti- C. albicans-specific antibody response similar to that induced by NAC. On the other hand, the anti-TNP antibody, used as a control, did not induce any alterations. In contrast, the late addition (after 1, 2 and 3 days) of anti-CD27 and anti-CD40 MoAbs to the antigen-stimulated cultures did not inhibit the induction of the specific antibody response (data not shown). Moreover, we performed CD40 cross-linking using a CD40L coated plate and found that the suppressive effects of 20 mm NAC were reversed in C. albicans-stimulated cultures (Table 2).
Fig. 5.
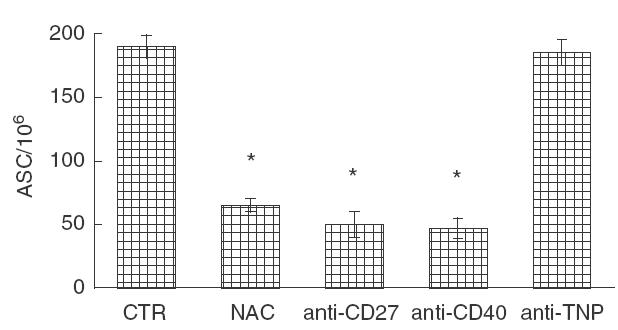
Effect of anti-CD27 and anti-CD40 MoAb addition on the induction of specific antibody-secreting cells (ASC). NAC 5 mm or anti-CD-27 (1 μg/ml) or anti-CD40 (1 μg/ml) or an irrelevant anti-2,4,6-trinitrophenyl (TNP) antibody were added to the C. albicans stimulated-culture. At day 9 cells were assayed for anti- Candida ASC. The data are expressed as mean ± s.e.m. from three independent experiments. * P <0·05 versus control.
Table 2.
Effect of CD40 cross-linking on NAC-induced immunosuppression of anti- C. albicans- specific antibody response
| ASC/106 | |
|---|---|
| CTR | 250 |
| NAC 20 mm | 3 |
| CD40L | 265 |
| CD40L + NAC 20 mm | 245 |
After 9 days of culture cells were assayed for anti- C. albicans antibody-secreting cells (ASC). One representative experiment of three is shown.
NAC inhibits PWM-induced polyclonal Ig production
To further characterize the down-regulatory effect of NAC on the B cell response, we investigated its interference with the T cell-dependent polyclonal induction of Ig production in PWM-stimulated cultures of human PBMC. The results represented in Fig. 6 show that NAC added at the beginning of culture significantly inhibited (P <0·05) PWM-dependent polyclonal Ig production at all concentrations used. Moreover, the addition of an anti-CD27 MoAb caused a significant decrease in the percentage of B cells producing intracellular total Ig whereas the addition of an anti-TNP antibody, used as a control, did not induce any effect.
Fig. 6.
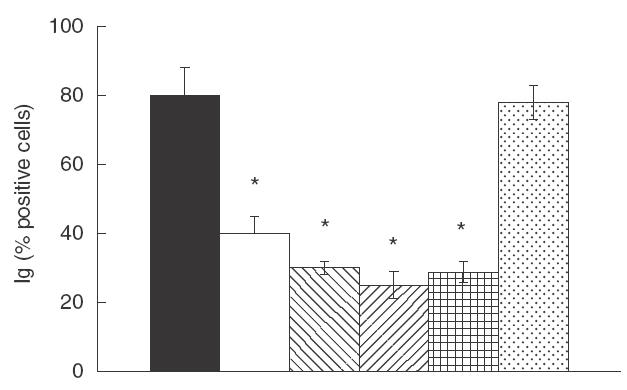
Effect of NAC and anti-CD27 addition on PWM stimulated-cultures. PWM-induced PBMC were cultured with increasing amounts of NAC or anti-CD27 (1 μg/ml) or an irrelevant anti-TNP antibody for seven days at 37°C. Intracellular Ig production was analysed by FACS. Results are expressed as percentage of Ig-positive cells. The data are expressed as mean ± s.e.m. from three independent experiments. * P <0·05 versus control. ▪, CTR; □, NAC 5mm;  , NAC 10 mm;
, NAC 10 mm;  , NAC 20 mm;
, NAC 20 mm;  , anti-CD27;
, anti-CD27;  , anti-TNP.
, anti-TNP.
NAC down-regulates membrane expression of co-stimulatory molecules on PWM-stimulated B cells
Because co-stimulatory signals are required both for PWM-driven B cell Ig synthesis and for induction of a specific antibody response to T cell-dependent antigens, we analysed whether NAC interfered with the expression of co-stimulatory molecules on the B cell surface in PWM-stimulated cultures. NAC induced an early (18 h) dose-dependent down-regulation of the surface expression of both CD40 and CD27 molecules on PWM-stimulated CD20+ cells (Fig. 7). Similar results were obtained after 48 and 72h of culture (data not shown). It should also be pointed out that NAC down-regulated the expression of CD40 and CD27 molecules on CD20− cells.
Fig. 7.
Effect of NAC on the surface expression of co-stimulatory molecules in PWM-activated B cells. Increasing amounts of NAC were added to PWM-stimulated cultures. After 18h cells were harvested and two-colour analysis was carried out staining with FITC-CD40 and PE-CD20 (left panel) or FITC-CD27 and PE-CD20 (right panel). Data are showed as density plots with green (FITC) fluorescence for CD40 and CD27 on the x-axis and orange (PE) fluorescence for CD20 on the y-axis in logarithmic scale. The percentage of double-positive cells is indicated. The data are from one representative experiment of three performed.
NAC does not inhibit T cell-independent SAC-driven B cell Ig production
Because PWM-driven B cell Ig synthesis is a complicated system, involving monocytes, B-cells and T cells, and because PWM is known to stimulate all these cells, we used the T cell-independent SAC-driven system of Ig production in order to clarify the effect of NAC on B cell Ig production. Figure 8 shows that the addition of NAC at the beginning of culture did not induce any modulation of the Ig production induced by SAC + IL-2 at all concentrations used. Moreover, the addition of anti-CD27 MoAb did not induce any modulation of Ig production. Similar results were obtained when adding an anti-TNP antibody, used as a control, in the same culture conditions. Finally, 20 mm NAC down-regulated the expression of CD40 and CD27 on B cells stimulated with SAC + IL-2 (data not shown).
Fig. 8.

Effect of NAC and anti-CD27 addition on SAC + IL-2-stimulated cultures. Increasing amounts of NAC or anti-CD27 (1 μg/ml) or an irrelevant anti-TNP antibody were added to SAC + IL-2-stimulated B cells. At day 7, intracellular Ig production was analysed by FACS. Results are expressed as percentage of Ig positive cells. The data are expressed as mean ± s.e.m. from three independent experiments. ▪, CTR; □, NAC 5 mm;  , NAC 10 mm;
, NAC 10 mm;  , NAC 20 mm;
, NAC 20 mm;  , anti-CD27;
, anti-CD27;  , anti-TNP.
, anti-TNP.
NAC modulates cytokine production
T cell-dependent B cell responses require both physical cell–cell contacts and cytokines to drive the different steps in B cell differentiation. Based on the observation that NAC was able to inhibit T cell-dependent anti- C. albicans antibody production by down-regulating the expression of co-stimulatory molecules on the B cell surface, we tested whether NAC inhibited, in these cultures, the production of IL-4 which is a key cytokine for B cell activation. We found that NAC induced a significant down-regulation of IL-4-SC and a significant up-regulation of IFN-γ-SC (Fig. 9).
Fig. 9.
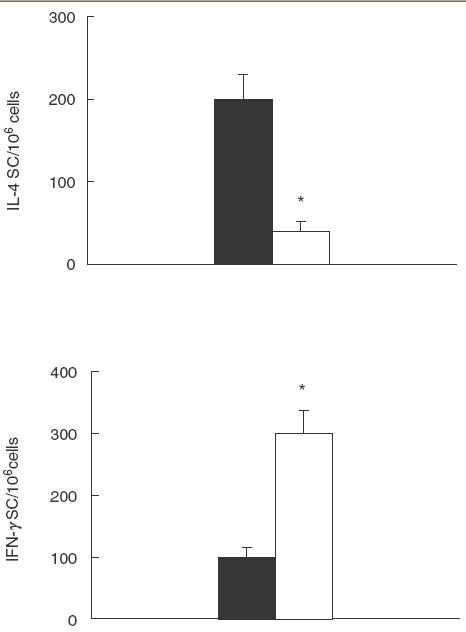
Effect of NAC on IL-4 and IFN-γ production. C. albicans-stimulated PBMC were cultured with or without NAC (5 mm). After 3 days, cultures were assayed for IL-4-SC and IFN-γ-SC. The data are expressed as mean ± s.e.m. from three independent experiments. * P <0·05 versus control. ▪, CTR; □, NAC.
DISCUSSION
In the present study, we show that NAC significantly inhibits the induction of the specific antibody response to the T cell-dependent C. albicans antigen by down-regulating both expression of CD40 and CD27 co-stimulatory molecules on the B cell surface and IL-4 production.
CD40 engaging CD40L on the T cell surface transduces activation signals required for the up-regulation of CD4+ T cell responses [21] and plays a central role in T cell-mediated B cell activation [22]. The CD27 molecule is expressed by a subset of B cells [23] that appear to be involved primarily in B cell regulation by enhancing Ig production. In contrast to CD40, CD27 signalling plays a minor role in B cell proliferation and its major function, which is delayed during the process of B cell differentiation, is to contribute to the formation of Ig producing cells [24].
We found that NAC added at the beginning of culture caused significant inhibition of the induction of C. albicans-specific ASC, while no inhibitory effect was evident at late time-points. This result indicates that NAC could interfere early in the activation pathway leading to the induction of a specific antibody response. Moreover, we found that the NAC-mediated inhibition of the specific antibody response was accompanied consistently by an early down-regulation of CD27 and CD40 co-stimulatory molecule expression on the B cell surface and that the addition of either anti-CD27 or anti-CD40 MoAbs, at the beginning of culture, also down-regulated the specific response while the late addition of the antibodies did not induce any effect. These results give further support to the notion that NAC immunosuppressive activity on the induction of an anti- C. albicans antibody response may be mediated by a mechanism involving down-regulation of the co-stimulatory molecules on the B cell surface. Kobata et al. (25) demonstrated that the interactions among subsets of CD4+ T cells expressing CD27 and its ligand CD70 play a key role in regulating B cell activation and immunoglobulin synthesis. We found that NAC also down-regulated the expression of CD40 and CD27 molecules on CD20− cells. In particular, we found that the expression of CD27 was reduced to less than 86% on the surface of CD4+ cells (data not shown) while the expression of CD40 was reduced to less than 85% on the surface of CD14+ (data not shown). Moreover, we found that the expression of CD70 (CD27 ligand) was reduced to less than 45% by NAC (data not shown). Therefore, NAC down-regulates both expression of CD27 and CD40, two key molecules involved in B cell function, on the B cell surface and CD27 and CD70, which are involved in helper/ suppressor T cell activity, on the T cell surface. NAC also inhibits T dependent B cell responses. Indeed, we can hypothesize that NAC exerts its effect on the B cell population both directly (down-regulating CD40 and CD27) and indirectly (down-regulating CD27 and CD70 expression on T cells).
To characterize further the down-regulatory effects of NAC on the B cell response, we investigated its interference with the induction of T cell-dependent polyclonal immunoglobulin production in PWM-stimulated cultures of human PBMC. We showed that NAC inhibited total Ig production by down-regulating the expression of CD40 and CD27 co-stimulatory molecules on the B-cell surface, confirming their central role in B cell activation.
Because the T cell-independent B cell activation system does not require signals delivered by T cell–B cell contact, we analysed the effect of NAC on T-independent SAC plus IL-2 induced polyclonal Ig production for a direct comparison [26]. We found that NAC did not induce any inhibition of Ig production, despite its down-regulatory effect on CD27 and CD40 expression on the B cell surface. This is consistent with the fact that the inhibitory effect of NAC is strongly dependent on co-stimulatory signals delivered by T cells.
As mentioned previously, T cell-dependent B cell responses, in addition to the necessary physical cell–cell contact, require activation signals delivered by cytokines with both autocrine and paracrine properties. We found that NAC significantly down-regulated IL-4 production by C. albicans-stimulated PBMC. Our results are in agreement with Jeannin et al. [11], who observed that NAC decreases IL-4 production by PMA/ionomycin or ConA-stimulated T cells. Moreover, Eylar et al. [27] reported that NAC suppresses IL-4 production induced by anti-CD3 and anti-CD28 in both control and HIV+ CD4+ T cells. IL-4 is an anti-inflammatory cytokine that exhibits multiple immunomodulatory functions on a variety of cell types, including T cells, B cells and monocytes [28]. Regarding B lymphocytes IL-4 increases cell viability, cell growth and up-regulates the expression of surface antigens, resulting in the enhancement of the antigen-presenting capacity of B cells [28]. Moreover, a key function of IL-4 is its ability to induce IgE and IgG4 production by human B cells [29]. Therefore, the inhibitory effect of NAC on antigen-specific Ig production might be the functional consequence of the down-regulation of IL-4 production induced by NAC.
On the other hand, we have shown a threefold increase in the number of IFN-γ secreting cells after NAC treatment. The relevant increase in the Th1-derived cytokine IFN-γ suggests that NAC may modulate Th1 and Th2 responses leading to the inhibition of humoral responses and a bias towards cell-mediated immunity. Indeed, a Th1 response may favour cell-mediated immunity, whereas a Th2 response provides help for humoral responses, including IgE and IgG.
Altogether these results indicate that in planning therapeutic strategies employing NAC it is important to consider its effects on the homeostasis of the immune system.
Acknowledgments
This work was supported by National Research Project on AIDS to MV and WM.
REFERENCES
- 1.Aruoma OIS, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Rad Biol Med. 1989;6:593–7. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 2.Staal FJT, Roederer M, Herzenberg LA. Intracellular thiols regulate activation of nuclear factor kB and transcription of human deficiency virus. Proc Natl Acad Sci USA. 1989;87:9943–7. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Look MP, Rockstroh JK, Rao GS, et al. Sodium selenite and N-acetylcysteine in antiretroviral-naive HIV-I-infected patients: a randomized, controlled pilot study. Eur J Clin Invest. 1998;28:389–97. doi: 10.1046/j.1365-2362.1998.00301.x. [DOI] [PubMed] [Google Scholar]
- 4.Muller F, Svardal AM, Nordoy I, Berge RK, Aukrust P, Froland SS. Virological and immunological effects of antioxidant treatment in patients with HIV infection. Eur J Clin Invest. 2000;30:905–14. doi: 10.1046/j.1365-2362.2000.00727.x. [DOI] [PubMed] [Google Scholar]
- 5.Sandstrom PA, Mannie MD, Buttke TM. Inhibition of activation-induced death in T cell hybridomas by thiol antioxidants: oxidative stress as a mediator of apoptosis. J Leuk Biol. 1994;55:221–6. doi: 10.1002/jlb.55.2.221. [DOI] [PubMed] [Google Scholar]
- 6.Yim CY, Hibbs JB, McGregor JR, Galinsky RE, Samlowski WE. Use of N-acetylcysteine to increase intracellular glutathione during the induction of antitumor responses by IL-2. J Immunol. 1994;152:5796–805. [PubMed] [Google Scholar]
- 7.Eylar E, Rivera-Quinones C, Molina C, Baez I, Molina F, Mercado CM. N-acetylcysteine enhances T cell functions and T cell growth in culture. Int Immunol. 1993;5:97–101. doi: 10.1093/intimm/5.1.97. [DOI] [PubMed] [Google Scholar]
- 8.Bengtsson A, Lundberg M, Avila-Carino J, Jacobsson G, Holmgren A, Scheynius A. Thiols decrease cytokine levels and down-regulate the expression of CD30 on human allergen-specific T helper (Th) 0 and Th2 cells. Clin Exp Immunol. 2001;123:350–60. doi: 10.1046/j.1365-2249.2001.01453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malorni W, D'Ambrosio A, Rainaldi G, Rivabene R, Viora M. Thiol supplier N-acetylcysteine enhances conjugate formation between natural killer cells and K562 or U937 targets but increases the lytic function only against the latter. Imm Lett. 1994;43:209–14. doi: 10.1016/0165-2478(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 10.Verhasselt V, Vanden Berghe W, Vanderheyde N, Willems F, Haegeman G, Goldman M. N-acetyl-l-cysteine inhibits primary human T cell responses at the dendritic cell level: association with NF-κB inhibition. J Immunol. 1999;162:2569–74. [PubMed] [Google Scholar]
- 11.Jeannin P, Delneste Y, Lecoanet-Hechoz S, et al. Thiol decrease human interleukin (IL) 4 production and IL-4-induced immunoglobulin syntesis. J Exp Med. 1995;182:1785–92. doi: 10.1084/jem.182.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanagihara Y, Basaki Y, Kajiwara K, Ikizawa K. A thiol antioxidant regulates IgE isotype switching by inhibiting activation of nuclear factor-κB. J Allergy Clin Immunol. 1997;100:33–8. doi: 10.1016/s0091-6749(97)70002-2. [DOI] [PubMed] [Google Scholar]
- 13.Torosantucci A, Palma C, Boccanera M, Ausiello CM, Spagnoli GC, Cassone A. Lymphoproliferative and cytotoxic responses in cultures of human peripheral blood mononuclear cells to mannoprotein constituents of Candida albicans. J Gen Microbiol. 1990;136:2155–63. doi: 10.1099/00221287-136-11-2155. [DOI] [PubMed] [Google Scholar]
- 14.Cassone A, De Bernardis F, Ausiello CM, et al. Immunogenetic and protective Candida albicans constituents. Res Immunol. 1998;149:289–99. doi: 10.1016/s0923-2494(98)80753-0. [DOI] [PubMed] [Google Scholar]
- 15.Luzzati AL. Induction of a specific antibody response in cultures of human peripheral blood lymphocytes. In: Lefkovits J, Pernis B, editors. Immunological methods. London: Academic Press; 1981. pp. 241–6. [Google Scholar]
- 16.Giacomini E, Boccanera M, Giordani L, Cassone A, Luzzati AL. Induction of antibody forming cells with specificity for Candida albicans mannoproteins in cultures of human peripheral blood lymphocytes. J Immunol Meth. 1993;164:203–11. doi: 10.1016/0022-1759(93)90313-v. [DOI] [PubMed] [Google Scholar]
- 17.Sedgwick JD, Holt PG. ELISA-plaque assay for the detection of single antibody-secreting cells. In: Kemeny DM, Challacombe SJ, editors. ELISA and other solid phase immunoassays. Chichester: Wiley; 1988. pp. 241–63. [Google Scholar]
- 18.Versteegen JMT, Logtemberg T, Ballieux RE. Enumeration of IFN-γ producing lymphocytes by spot-ELISA. A method to detect lymphokine-producing lymphocytes at the single cell level. J Immunol Meth. 1988;111:25–9. doi: 10.1016/0022-1759(88)90055-5. [DOI] [PubMed] [Google Scholar]
- 19.Barbieri D, Grassilli E, Monti D, et al. d-ribose and deoxy-d-ribose induce apoptosis in human quiescent peripheral blood mononuclear cells. Biochem Biophys Res Commun. 1994;201:1109–16. doi: 10.1006/bbrc.1994.1820. [DOI] [PubMed] [Google Scholar]
- 20.Giordani L, Giacomini E, Quaranta MG, Viora M. HIV-nef protein inhibits the in vitro induction of a specific antibody response to Candida albicans by an early up-regulation of IL-15 production. Clin Exp Immunol. 2000;122:358–63. doi: 10.1046/j.1365-2249.2000.01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grewal IS, Flavell RA. A central role of CD40-ligand in the regulation of CD4+ T-cell responses. Immunol Today. 1996;17:410–4. doi: 10.1016/0167-5699(96)10030-x. [DOI] [PubMed] [Google Scholar]
- 22.Nishioka Y, Lipsky PE. The role of CD40–CD40 ligand interaction in human T cell–B cell collaboration. J Immunol. 1994;153:1027–36. [PubMed] [Google Scholar]
- 23.Maurer D, Holter W, Majdic O, Fischer GF, Knapp W. CD27 expression by a distinct subpopulation of human B lymphocytes. Eur J Immunol. 1990;20:2679–84. doi: 10.1002/eji.1830201223. [DOI] [PubMed] [Google Scholar]
- 24.Jacquot S, Kobata T, Iwata S, Morimoto C, Schlossman SF. CD154/CD40 and CD70/CD27 interactions have different and sequential functions in T cell-dependent B cell responses. J Immunol. 1997;159:2552–7. [PubMed] [Google Scholar]
- 25.Kobata T, Jacquot S, Kozlowski S, Agematsu K, Schlossman SF, Morimoto C. CD27–CD70 interactions regulate B-cell activation by T cells. Proc Natl Acad Sci USA. 1995;92:11249–53. doi: 10.1073/pnas.92.24.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuurman RK, Gelfand EW, Dosch HM. Polyclonal activation of human lymphocytes in vitro. I. Characterisztion of the lymphocyte response to a Tcell-independent B cell mitogen. J Immunol. 1980;125:820–6. [PubMed] [Google Scholar]
- 27.Eylar EH, Baez I, Vazquez A, Yamamura Y. N-acetylcysteine (NAC) enhances interleukin-2 but suppress inteleukin-4 secretion from normal and HIV+CD4+T-cells. Cell Mol Biol. 1995;41 (Suppl.1):S35–40. [PubMed] [Google Scholar]
- 28.Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–70. [PubMed] [Google Scholar]
- 29.Pene J, Rousset F, Briere F, et al. IgE production by normal human lymphocytes is induced by IL-4 and suppressed by α-interferon and prostaglandin E2. Proc Natl Acad Sci USA. 1988;85:6880–4. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]


