Abstract
Diets enriched in n-3 polyunsaturated fatty acids (PUFA) suppress several functions of murine splenic T cells by acting directly on the T cells and/or indirectly on accessory cells. In this study, the relative contribution of highly purified populations of the two cell types to the dietary suppression of T cell function was examined. Mice were fed diets containing different levels of n-3 PUFA; safflower oil (SAF; control containing no n-3 PUFA), fish oil (FO) at 2% and 4%, or 1% purified docosahexaenoic acid (DHA) for 2 weeks. Purified (>90%) T cells were obtained from the spleen, and accessory cells (>95% adherent, esterase-positive) were obtained by peritoneal lavage. Purified T cells or accessory cells from each diet group were co-cultured with the alternative cell type from every other diet group, yielding a total of 16 different co-culture combinations. The T cells were stimulated with either concanavalin A (ConA) or antibodies to the T cell receptor (TcR)/CD3 complex and the costimulatory molecule CD28 (αCD3/αCD28), and proliferation was measured after four days. Suppression of T cell proliferation in the co-cultures was dependent upon the dose of dietary n-3 PUFA fed to mice from which the T cells were derived, irrespective of the dietary treatment of accessory cell donors. The greatest dietary effect was seen in mice consuming the DHA diet (P = 0·034 in the anova; P = 0·0053 in the Trend Test), and was observed with direct stimulation of the T cell receptor and CD28 costimulatory ligand, but not with ConA. A significant dietary effect was also contributed accessory cells (P = 0·033 in the Trend Test). We conclude that dietary n-3 PUFA affect TcR-mediated by T cell activation by both direct and indirect (accessory cell) mechanisms.
Keywords: docosahexaenoic acid, lymphocyte, cell proliferation
INTRODUCTION
Dietary n-3 polyunsaturated fatty acids (PUFA), e.g. eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), found in fish oil (FO) have been shown to decrease T cell proliferation [1,2], cytokine secretion [3–6], intracellular enzyme activity [7,8], and gene transcription [4,9] in humans and experimental animals. Previous studies have examined the effects of n-3 PUFA on mixed cell populations (e.g. whole splenocytes) [1,4,10,11], purified murine T cells [12–14], or immortalized T cell lines [15,16].
We previously reported [12], that purified splenic T cells taken from mice fed diets containing 1% DHA produced less Interleukin (IL)-2 when stimulated at the plasma membrane receptor level with antibodies to CD3 and CD28, than T cells from mice fed 1% arachidonic acid (AA), an n-6 PUFA. Thus, in our experimental system, dietary fatty acids appear to modulate T-cell plasma membrane dynamics and alter T cell receptor (TcR)-dependent or costimulatory receptor (CD28) signal transduction. We demonstrated further that dietary n-3 PUFA mediate agonist-specific effects on T cell proliferation, that the effects differ with the phenotype of the purified T cells (i.e. CD4+ or CD8+), and that the effects are related to the resulting Th1 or Th2 cytokine profiles [17].
Accessory cells (e.g. macrophages, dendritic cells, B cells) play an important role in the propagation of an inflammatory response by processing and presenting antigen to T cells in the context of cell surface proteins encoded in the Class I and II genes of the major histocompatibility complex (MHC). In addition, accessory cells respond to the initial ligation and ingestion of foreign material by producing soluble mediators (e.g. IL-1, tumour necrosis factor alpha (TNFα) IL-10, transforming growth factor beta (TGFβ), nitric oxide, etc.) and up-regulating the expression of specific ligands such as B7·1 and B7·2, which collectively regulate T cell activation and function [18]. Dietary n-3 PUFA has been shown repeatedly to cause changes in the lipid composition of membranes from rat and mouse macrophages [19–21], and in the production of PGE, TNFa, and IL-1 [20,22–24]. Therefore, it is possible that dietary n-3 PUFA might modulate T cell activation indirectly, by affecting some aspects of accessory cell function.
The results of earlier macrophage-lymphocyte co-culture experiments in our laboratory had indicated that the principal effect of dietary n-3 PUFA appeared to be directly on suppressing the response of the lymphocytes to a polyclonal T cell mitogen, concanavalin A (ConA), as opposed to being mediated through modulation of accessory cell functions [14]. This lack of apparent contribution of the accessory cells from mice fed n-3 PUFA-enriched diets to suppression of T cell activation was surprising. Nonetheless, others have observed that macrophages from n-3 PUFA-fed animals did not differ from controls in their phagocytic activity, enzyme activities, or developmental state [25,26]. More importantly Calder et al. [27] have demonstrated that the presence or absence of adherent cells did not affect dietary n-3 PUFA-induced alterations in T cell responses.
While these results seem to support a direct effect of n-3 PUFA on T cell activation, most of these experiments were conducted with relatively heterogenous populations of splenic cells which probably contained endogenous accessory cells in addition to the exogenous accessory cells added from animals consuming a different diet. Furthermore, we recently published the results of a series of experiments using highly purified T cells from mice fed diets containing different levels of n-3 PUFA [12]. Proliferation of T cells was induced by combinations of agonists acting on T cell surface receptors (anti-CD3, anti-CD28) or directly on intracellular messengers (phorbol myristate acetate-PMA, ionomycin). Surprisingly, there was no diet effect on T cell proliferation with ConA as we had observed in whole spenocyte cultures [14,28]. Since these highly purified T cells did not obviously require accessory cells to be stimulated by the agonists employed, the absence of suppression by n-3 PUFA indicated that accessory cells may play an important ‘bystander’ role in dietary n-3 PUFA-mediated suppression of T cell activation in our murine model system.
Therefore, we undertook a series of experiments to examine the relative importance of the immunomodulatory effects of dietary n-3 PUFA mediated either by a direct effect of diet on T cell activation per se, an indirect effect of diet on accessory cell function, or both. We utilized co-cultures of highly purified T cells or accessory cells from mice fed four different diets and cultured together in all possible (16) factorial combinations.
MATERIALS AND METHODS
Diet and animals
All experimental procedures using laboratory animals were approved by the University Laboratory Animal Care Committee of Texas A & M University. Female, pathogen-free young (12–14 g) C57BL/6 mice were purchased from Frederick National Cancer Research Facility (Frederick, MD, USA). Animals were housed in groups of six in polycarbonate microisolator cages, had free access to autoclaved water and diet, and were maintained at room temperature (∼25°C) on a 12-h light : dark cycle. Mice were initially fed standard mouse chow (Teklad 9F Sterilizable Rodent diet, Madison, WI, USA) during a 1-week acclimation period and were subsequently assigned to one of four semipurified diets: 2% (by weight) safflower oil ethyl ester (SAF) plus 3% corn oil (CO) (control diet containing no n-3 PUFA); FO at 2% or 4% containing 3% and 1% CO, respectively; or 1% DHA ethyl ester plus 4% CO, for two weeks. Diets were analysed by gas chromatography prior to feeding, aliquoted and stored at −80°C, and changed daily to prevent peroxidation. The proximal analysis of identical diets has already been published by us [17]. The analysis confirmed the enrichment of 18 : 2 (n-6) in the SAF diet (64·1% of total fatty acids), and 20 : 5 (n-3) and 22 : 6 (n-3) in the DHA (0/18·3% of total fatty acids), 2% FO (5·9%/4·2% of total fatty acids), and 4% FO (13·5%/6·8% of total fatty acids) diets, respectively.
The purified diets met National Research Council nutrition requirements and varied only in lipid composition [29]. The basic diet composition (in g/kg), which has been published previously by us [17], was: 200 g casein, 420 g sucrose, 219·8 g starch, 60 g cellulose, 35 g AIN-76 mineral mix, 10 g vitamin mix AIN-76, 3 g DL-methionine, 2 g choline chloride, 0·2 g tertiary butyl hydroquinone, and 50 g of lipid. The four diet groups varied by PUFA lipid source only, containing 20 g/kg SAF plus 30 g/kg CO, or 20 g/kg or 40 g/kg Menhaden fish oil (FO) plus 30 or 10 g/kg CO, respectively, or 10 g/kg DHA ethyl ester plus 40 g/kg CO. The minimum linoleic acid (18 : 2 n-6) content from CO was 2·3% of total energy and thus met the minimum 1–2% requirement for rodents [29]. The vitamin E levels were approximately equal (mean ± SEM; 169·2 ± 4·4 mg/kg diet) and exceeded the minimum requirement (22 mg Vitamin E/kg diet) [29]. DHA (88·9% as 22 : 6 n-3) and SAF (70·5% as 18 : 2 n-6) were obtained in ethyl ester form from the National Institute of Health Test Materials Program (Charleston, SC, USA). Menhaden fish oil (13·1% as 20 : 5 n-3, 9·7% as 22 : 6 n-3) was provided by the National Institutes of Health Test Materials Program, and CO (57·3% as 18 : 2 n-6) was obtained from Degussa Bioactives (Champaign, IL, USA).
Isolation and preparation of purified T lymphocytes and accessory cells
Mice were killed via CO2 asphyxiation. Resident macrophages were isolated from the unstimulated mouse peritoneum by injecting the peritoneal cavity with Hank's buffered saline solution (Fisher Scientific, Houston, TX, USA) and washing twice. Cells were pooled and centrifuged at 800 ×g for 5 min, washed once, and the viability determined by trypan blue exclusion [30,31]. The purity of the cell population was determined to be greater than 90% esterase-positive, adherent cells. Spleens were placed in 3 ml of RPMI complete medium [(RPMI 1640 with 25 mm HEPES; Irvine Scientific, Santa Ana, CA, USA, supplemented with 10% FBS; Irvine Scientific, 1 × 105 U/l penicillin and 100 mg/l streptomycin (Irvine Scientific), 2 mm l-glutamine, and 10 µm 2-mercaptoethanol][11]. Spleens were dispersed with glass homogenizers and passed through a 149-micron wire mesh filter to create single-cell suspensions. Splenocytes were washed with RPMI complete medium prior to T lymphocyte enrichment.
T lymphocyte enrichment
Total lymphocytes were initially enriched by density gradient centrifugation using Lympholyte-M (Cedarlane, Toronto, Ontario, Canada) in accordance with the manufacturer's protocol. Subsequently, 60–90 × 106 mononuclear cells were loaded onto a negative selection mouse T cell purification column (R & D Systems, Minneapolis, MN, USA) and incubated for 10 min at room temperature. Nonadherent cells were eluted for purity analysis, co-culture and proliferation assays. The purity of the T cell population was analysed by flow cytometry (FACScan; Becton-Dickenson, Bedford, MA, USA) as previously described by Darzynkiewicz and Crissman [32] using anti-CD3 antibody conjugated to fluorescein isothiocyanate (PharMingen, San Diego, CA, USA), and determined to be 90·3 ± 1·4% (n = 4).
Co-culture and T-lymphocyte proliferation assay
Preliminary experiments were performed with co-cultures containing different ratios of purified T lymphocytes and purified accessory cells to determine the ratio and total cell number which supported optimal proliferative responses to the two stimuli employed in these experiments. Co-cultures containing a final ratio of 16 T lymphocytes per accessory cell in a total of 1 × 105 cells per well were established in 96-well plates. Every combination of T lymphocytes from the four diet groups with accessory cells from the four diet groups yielded 16 different co-culture combinations. Triplicate wells were stimulated with either 1 µg/ml plate-bound purified hamster antimouse CD3e (αCD3) monoclonal antibody (Pharmingen, San Diego, CA, USA) with 5 µg/ml soluble purified hamster antimouse CD28 (αCD28) monoclonal antibody (Pharmingen) or with the polyclonal T cell mitogen, concanavalin A at 2·5 µg/ml (ConA; Sigma Chemical, St Louis, MO, USA). These concentrations were determined by preliminary proliferation assays using various doses to induce proliferation without compromising viability (>90% viable cells) [17].
Cells were incubated at 37°C in an atmosphere of 5% CO2 in air for 72 h. For the final 6 h, 1·0 µCi [3H]-thymidine/well (New England Nuclear, North Bellerica, MA, USA) was added to the cultures. Cells were harvested onto glass fibre filter paper discs (Whatman, Maidstone, UK) using a multiple automated sample harvester unit (MASH II; MA Bioproducts, Walkersville, MD, USA). Cellular uptake of [3H]-thymidine was measured using a liquid scintillation counter (LS 8000, Beckman Instruments, Irvine, CA, USA). Results are expressed as the net disintegrations per minute (DPM) (stimulated minus control) of triplicate cultures [14].
Statistical analysis
A linear mixed model [33] that incorporated the correlation structure and random effects of individual mice was developed. The individual mice were preserved as the experimental units through the correlational structure of the data. There were 16 possible combinations–4 T cell diet types times 4 macrophage diet types with 4–5 mice per combination. To account for the fact that individual animals contributed either T cells or macrophages to multiple combinations, we developed a model for the correlations. The result was a standard linear mixed effects model, which was fit using the restricted maximum likelihood option [34]. Using this procedure, hypothesis testing was performed for main effects and interactions [35]. The Trend Test was implemented instead of the F-test to determine main effects. The Trend Test is appropriate when an a prior hypothesis exists that the population means should be in the same order as the amount of DHA in the diet, which is used as a categorical factor in this case. PTREND less than 0·05 implies that there is a statistically significant dose–response within the cell type. Pairwise comparisons among the different combinations of T cells and macrophages were made using the Least Significant Difference test.
RESULTS
There was no significant difference in food intake between dietary groups and final body weights (mean ± SEM) were: SAF diet, 20·60 g ± 0·22 g; DHA diet, 22·40 g ± 0·41 g; 2% FO diet, 20·48 g ± 0·63 g; 4% FO diet, 19·32 ± 0·53; n = 12. DHA-fed mice gained significantly more weight (P < 0·05) than the other three diet groups, which did not differ statistically from each other. Table 1 reveals the effect of dietary n-3 PUFA on the proliferative responses of homologous co-cultures (i.e., both accessory cells and T lymphocytes taken from the same diet group) to the two in vitro stimuli. It is clear that dietary n-3 PUFA, especially DHA, had a suppressive effect on the proliferative response of purified T lymphocytes following direct stimulation of the T cell receptor and coreceptor (αCD3/αCD28). The diet enriched with DHA resulted in nearly a 50% reduction in proliferation of homologous co-cultures stimulated with the antibodies. In contrast, diet exerted no significant effect on ConA-induced proliferation in the same co-cultures.
Table 1.
Proliferative responses to in vitro stimuli of homologous co-cultures of purified T lymphocytes and accessory cells taken from the same diet group
| T cell Proliferation (dpm)† | ||
|---|---|---|
| Dietary treatment* | αCD3/αCD28 | ConA |
| SAF | 92 590 ± 8832a | 25 757 ± 2156a |
| FO (2%) | 83 020 ± 15 534a | 27 548 ± 1591a |
| FO (4%) | 87 159 ± 9471a | 34 717 ± 7200a |
| DHA | 56 755 ± 5574b | 29 804 ± 5668a |
SAF, 2% safflower oil control diet containing no n-3 PUFA; FO (2%), 2% fish oil; FO (4%), 4% fish oil; DHA, 1% DHA ethyl ester.
Uptake of 3H-TdR after 78 h in culture with either 1 µg/ml plate-bound anti-CD3 and 5 µg/ml soluble anti-CD28 (αCD3/αCD28) or concanavalin A (2·5 µg/ml).
Mean ± SEM (n = 3–5 mice) in the same column designated by different superscript letters are significantly different (P < 0·05).
The differential effect of dietary n-3 PUFA on the proliferation induced by αCD3/αCD28 in heterologous co-cultures, that is, co-cultures containing purified T lymphocytes and accessory cells from different diets, is illustrated in Fig. 1. A total of 16 different co-culture conditions were examined. Each co-culture contained lymphocytes from one of the diet groups (i.e. LS, LD, LF2, LF4) combined with accessory cells (macrophages) from each of the diet groups (i.e. (M)S (M)D (M)F2 (M)F4) in a factorial 4 × 4 design. T lymphocytes from SAF-fed mice (LS) proliferated to the same degree regardless of the dietary treatment of the accessory cell donors [(M)S (M)D (M)F2 (M)F4]. There was no significant effect of accessory cell source on the proliferation of lymphocytes from animals fed the 2% FO diet (LF2), however, lymphocytes from the 4% FO group (LF4) were suppressed significantly when they were co-cultured with accessory cells from the DHA group [(M)D]. When all of the T cell responses for each diet were pooled across all accessory cell sources, there was a highly significant dietary effect (PTREND = 0·0053). These results are illustrated in Fig. 2, which plots the T cell responses for each diet group in combination with all macrophage diet sources, and indicates that the αCD3/αCD28-induced proliferation of lymphocytes from the 4% FO and DHA groups was significantly suppressed in comparison to cells from the SAF and 2% FO diets, regardless of accessory cell source (PTREND < 0·05). Likewise, as shown in Fig. 3, when a similar analysis was performed for αCD3/αCD28-induced T cell proliferation data for each macrophage diet source, pooled across all T lymphocyte dietary sources, there was a significant dietary effect (PTREND = 0·0326), with macrophages from the DHA-fed mice having the most significant suppressive effect on T cells regardless of the diet fed to the T cell donors.
Fig. 1.

The in vitro proliferative response to αCD3/αCD28 of co-cultures of purified T lymphocytes (L) and purified accessory cells (M) isolated from mice fed diets containing different levels of n-3 PUFA for two weeks; lymphocytes from each diet source (LS, LD, LF2, LF4) were co-cultured with accessory cells from each diet source (▪ (M)S, (M)D,
(M)D, (M)F2, □ (M)F4) in a 4 × 4 factorial design; S, Safflower oil diet; F2, 2% Fish oil diet; F4, 4% Fish oil diet; D, DHA-enriched diet; Mean ± SEM (n = 3–5 mice).
(M)F2, □ (M)F4) in a 4 × 4 factorial design; S, Safflower oil diet; F2, 2% Fish oil diet; F4, 4% Fish oil diet; D, DHA-enriched diet; Mean ± SEM (n = 3–5 mice).
Fig. 2.
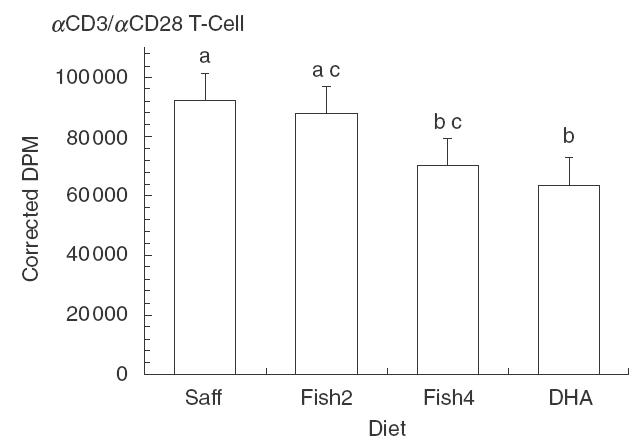
Lymphocyte diet source as a determinant of lymphoproliferation induced by αCD3/αCD28 in cultures of purified T cells from mice fed four different levels of n-3 PUFA in co-culture with purified accessory cells from all four diet sources; Saff, safflower oil diet; Fish2, 2% fish oil diet; Fish4, 4% fish oil diet; DHA DHA-enriched diet; mean ± SEM for n = 12–18 mice; values identified by a different letter are significantly different (PTREND < 0·05).
Fig. 3.

Accessory cell diet source as a determinant of lymphoproliferation induced by αCD3/αCD28 in cultures of accessory cells from mice fed four different levels of n-3 PUFA in co-culture with purified T lymphocytes from all four diet groups; Saff, safflower oil diet; Fish2, 2% fish oil diet; Fish4, 4% fish oil diet; DHA, DHA-enriched diet; mean ± SEM for 12–18 mice; values identified by a different letter are significantly different (PTREND < 0·05).
The differential effect of dietary n-3 PUFA on the proliferation induced by ConA in heterologous co-cultures, i.e. co-cultures containing purified T lymphocytes and accessory cells from different diets, is illustrated in Fig. 4. As can be appreciated, there was no statistically significant effect of diet on the proliferation of T lymphocytes to this polyclonal mitogen regardless of the dietary source of the T lymphocytes or accessory cells in the co-cultures. Unlike the response to αCD3/αCD28 described above, none of the co-culture combinations resulted in significant alterations of T cell proliferative responses even when cells were derived from the DHA-fed mice. A similar analysis by the Trend Test of overall diet effects on either cell type, summed across all diets for the other cell type in the co-culture, resulted in no signficant values.
Fig. 4.
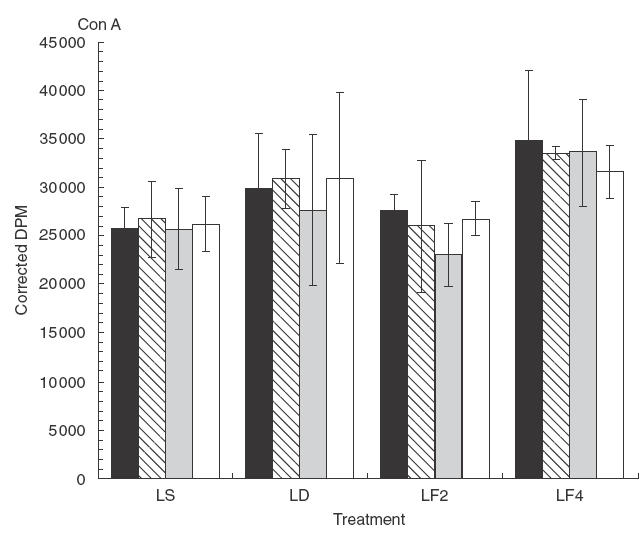
The in vitro proliferative response to ConA of co-cultures of purified T lymphocytes (L) and purified accessory cells (M) isolated from mice fed diets containing different levels of n-3 PUFA for two weeks; lymphocytes from each diet source (LS, LD, LF2, LF4) were co-cultured with accessory cells from each diet source (▪ (M)S, (M)D,
(M)D, (M)F2, □ (M)F4) in a 4 × 4 factorial design; S, Safflower oil diet; F2, 2% Fish oil diet; F4, 4% Fish oil diet; D, DHA-enriched diet; Mean ± SEM (n = 3–5 mice); no statistically significant dietary effects were detected.
(M)F2, □ (M)F4) in a 4 × 4 factorial design; S, Safflower oil diet; F2, 2% Fish oil diet; F4, 4% Fish oil diet; D, DHA-enriched diet; Mean ± SEM (n = 3–5 mice); no statistically significant dietary effects were detected.
DISCUSSION
Dietary n-3 PUFA have a profound effect on several aspects of T lymphocyte activation, including proliferation and cytokine production, in both humans and animal models, as shown by us and others [1,3–5,11,12,14,17]. That these effects clearly are mediated, at least in part, by the direct impact of diets enriched in n-3 PUFA on T lymphocytes themselves is supported by at least three lines of evidence. First, we and others have demonstrated that the membranes of murine T cells are remodeled, quantitatively and qualitatively, by the fatty acid content of the diet, which is reflected in the lipid composition of T lymphocyte membranes [16,36,37]. Second, T cell proliferation following direct stimulation of the TcR/CD3 complex and the costimulatory ligand, CD28, is suppressed in the absence of accessory cells [12]. Third, diets rich in n-3 PUFA result in enhancement of activation-induced apoptosis in T lymphocytes in an accessory cell-independent fashion [36].
However, there is also good evidence that dietary n-3 PUFA affect accessory cell (principally macrophage) membrane lipid composition and cytokine production [19–24]. We and others have shown that the n-3 PUFA-associated T cell suppression appears not to be mediated through effects on accessory cells [3,12,14,17]. Some of these previous studies were carried out with relatively heterogeneous, unseparated populations of splenocytes which likely contained endogenous accessory cells subjected to the same diet as the lymphocytes [14], while other studies employed in vitro stimuli which are accessory cell-independent [12,17]. In that regard, the fact that the homologous co-cultures of T cells and accessory cells taken from mice consuming the same diets (which may approximate the unseparated cell populations used in earlier studies) showed a clear n-3 PUFA-associated suppression of proliferation to stimulation with αCD3/αCD28 (Table 1) confirms the biological relevance of our co-culture system.
The results presented here are unique in that they were derived from co-cultures of highly purified T lymphocytes and adherent macrophages from mice fed diets containing different levels of n-3 PUFA. The 4 × 4 factorial study design allowed us to examine the effect of dietary n-3 PUFA on purified T lymphocytes from each diet group in co-culture with purified accessory cells from each diet group. This yielded a total of 16 different co-culture conditions. The analysis was performed to test the independent effects of diet on lymphocyte source and macrophage source, and permitted a significant dietary effect of both cell sources on lymphoproliferation to be demonstrated. The results reveal that dietary n-3 PUFA suppressed T lymphocyte prolilferation directly (see Fig. 2), regardless of the dietary source of accessory cells, and indirectly (see Fig. 3) by its effects on accessory cells. These results are not contradictory, rather, they suggest that n-3 PUFA are acting to modulate the functions of both cell populations to influence lymphoproliferation by different mechanisms.
The DHA diet induced the development of macrophages which significantly suppressed the proliferation of T cells, irrespective of the diet consumed by the T cell donors (Fig. 3). This accessory cell-dependent effect was seen only when the lymphocytes were stimulated via the TcR/CD3 complex in association with the CD28 costimulatory molecule. Since αCD3/αCD28 treatment is capable of triggering T cell proliferation in the absence of exogenous accessory cells [12,17], the suppressive effects of macrophages from DHA-fed mice is likely mediated in a ‘by-stander’ fashion, that is, by the production of soluble ‘suppressor’ cytokines such as IL-10 and/or TGFβ. Future studies with the two cell populations separated physically from each other by a transwell insert, in combination with antibody treatment to block the effects of soluble cytokines, may clarify this issue.
The data also support a direct effect of diet on αCD3/αCD28-induced T cell proliferation irrespective of the diet consumed by the macrophage donors (Fig. 2). A statistically significant dietary effect on T cell proliferation was seen with both the 4% FO diet and the DHA diet, but not the 2% FO diet, implying that there may be a dose effect of n-3 PUFA in mediating direct suppression of T cell lymphoproliferation. The 22 : 6 (n-3) content of the DHA diet was 18·3% of total fatty acids, while that of the 4% FO diet was 6·8%. The 2% FO diet, by contrast, contained only 4·2% of total fatty acids as 22 : 6 (n-3). We have demonstrated recently that our n-3 PUFA-enriched diets result in significant remodeling of T lymphocyte membrane lipid compostion [36]. Therefore, we hypothesize that diet may alter the expression, integrity and/or signalling functions of the TcR/CD3 complex and/or CD28 in the membrane. In a previous study, we observed a differential diet effect on murine T cells polarized in vitro toward a Th1 or Th2 cytokine profile which appeared to vary according to the total amount of n-3 PUFA in the diets [17].
The significant effect of dietary n-3 PUFA on lymphoproliferation in co-cultures stimulated with αCD3/αCD28 was not seen when the same combinations of cells were incubated with the polyclonal T cell mitogen, concanavalin A (Fig. 4). This is a very important observation because the contrast between the effect of diet on these two stimuli may reveal some fundamental truths about the mechanism of n-3 PUFA action in our model. Thus, the results infer that the dietary effect required direct stimulation of the TcR/CD3 and CD28 molecules, and did not influence the response of purified T cells to a mitogenic ligand which binds to TcR/CD3 and to promiscuous glycoprotein receptors (e.g. CD2) on the surface of the cell, but not to CD28 [38]. Since ConA does not bind to CD28, and its stimulatory activity was not diminished by dietary n-3 PUFA in this study, the results suggest that alteration of signalling through the CD28 coreceptors is at least one of the mechanisms by which n-3 PUFA directly suppress T cell activation, as we have reported previously [12,17].
Both the homologous co-cultures (Table 1) and the mixed co-cultures proliferated to essentially the same degree in response to ConA, regardless of the dietary treatment of the donors of either cell type. Thus, the co-cultures did not reproduce the n-3 PUFA diet effect seen in ConA-stimulated cultures of whole spenocytes examined previously by us [1,11,14,28]. A likely explanation for this apparent discrepancy is that the co-cultures examined in the present study contained only purified T lymphocytes and purified adherent accessory cells. Whole splenocyte cultures, such as those utilized in the studies cited above, likely contained additional cell types (e.g. B lymphocytes, dendritic cells, etc.) which were not present in these co-cultures and which could be influencing the reponse of the T cells to ConA in unknown ways. An experimental approach to examining this interesting phenomenon would be to add purified splenic B cells or dendritic cells from n-3 PUFA-fed mice to the co-cultures to see what effect they might have on ConA-driven T cell activation.
In conclusion, the proliferative response of highly purified murine T lymphocytes to in vitro stimulation of the TcR/CD3 complex in association with the CD28 costimulatory molecule was suppressed, in a dose-dependent manner, by the DHA fed to donors of both T cells and purified accessory cells (principally macrophages) present in the co-cultures. T cell proliferation was suppressed significantly in co-cultures containing accessory cells from DHA-fed mice, regardless of the dietary treatment of the T cell donors. At the same time, lymphoproliferation was significantly suppressed in co-cultures containing T cells from DHA and 4% FO diet-fed donors, regardless of the source of accessory cells. These effects were not observed with ConA stimulation, which further supports our conclusion that the CD28 molecule is a primary target of the direct n-3 PUFA diet effect on T cell activation [12,17]. The precise mechanisms underlying the direct and indirect effects of dietary n-3 PUFA in this system, and the basis for the accessory cell contribution to these effects, remain to be elucidated. Future studies will address the issue of contact dependence, and the role of soluble cytokines (e.g. IL-10, TGFβ) which may be mediating the accessory cell effect.
Acknowledgments
The authors wish to thank Kirsten Switzer and Yang-Yi Fan for their valuable technical assistance in the completion of these studies.
Supported by NIH grants DK53055 (RSC), CA57030 (RJC), and P30-ES09106.
REFERENCES
- 1.Jolly CA, Laurenz JC, McMurray DN, et al. Diacylglycerol and ceramide kinetics in primary cultures of activated T-lymphocytes. Immunol Lett. 1996;49:43–8. doi: 10.1016/0165-2478(95)02486-7. [DOI] [PubMed] [Google Scholar]
- 2.Peterson LD, Jeffery NM, Thies F, et al. Eicosapentaenoic and docashexaenoic acids alter rat spleen leukocyte fatty acid composition and prostaglandin E2 production but have different effects on lymphocyte functions and cell-mediated immunity. Lipids. 1998;33:171–80. doi: 10.1007/s11745-998-0193-y. [DOI] [PubMed] [Google Scholar]
- 3.Calder PC. n-3 polyunsaturated fatty acids and cytokine production in health and disease. Ann Nutr Metab. 1997;41:203–34. doi: 10.1159/000177997. [DOI] [PubMed] [Google Scholar]
- 4.Fritsche LL, Byrge M, Feng C. Dietary omega-3 polyunsaturated fatty acids from fish oil reduce interleukin-12 and interferon-gamma production in mice. Immunol Lett. 1999;65:167–73. doi: 10.1016/s0165-2478(98)00109-6. [DOI] [PubMed] [Google Scholar]
- 5.Meydani SN, Endres S, Woods MM, et al. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121:547–55. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]
- 6.Virella G, Fourspring K, Hyman B, et al. Immunosuppressive effects of fish oil in normal human volunteers: correlation with the in vitro effects of eicosapentaenoic acid on human lymphocytes. Clin Immunol Immunopathol. 1991;61:161–76. doi: 10.1016/s0090-1229(05)80021-2. [DOI] [PubMed] [Google Scholar]
- 7.May CL, Southworth AJ, Calder PC. Inhibition of lymphocyte protein kinase C by unsaturated fatty acids. Biochem Biophys Res Comm. 1993;195:823–8. doi: 10.1006/bbrc.1993.2119. [DOI] [PubMed] [Google Scholar]
- 8.Speizer LA, Watson MJ, Brunton LL. Differential effects of omega-3 fish oils on protein kinase activities in vitro. Am J Physiol. 1991;261:E109–14. doi: 10.1152/ajpendo.1991.261.1.E109. [DOI] [PubMed] [Google Scholar]
- 9.Sellmayer A, Danesch U, Weber PC. Effects of different polyunsaturated fatty acids on growth-related early gene expression and cell growth. Lipids. 1996;31:S37–40. doi: 10.1007/BF02637048. [DOI] [PubMed] [Google Scholar]
- 10.Jenski LJ, Scherer JM, Caldwell LD, et al. The triggering signal dictates the effect of docosahexaenoic acid on lymphocyte function in vitro. Lipids. 1998;33:869–78. doi: 10.1007/s11745-998-0283-x. [DOI] [PubMed] [Google Scholar]
- 11.Jolly CA, Jiang YH, Chapkin RS, et al. Dietary (n-3) polyunsaturated fatty acids suppress murine lymphoproliferation, interleukin-2 secretion, and the formation of diacylglycerol and ceramide. J Nutr. 1997;127:37–43. doi: 10.1093/jn/127.1.37. [DOI] [PubMed] [Google Scholar]
- 12.Arrington JL, Switzer KC, Fan YY, et al. Docosahexaenoic acid suppresses function of the CD28 costimulatory membrane receptor in primary murine and Jurkat T cells. J Nutr. 2001;131:1147–53. doi: 10.1093/jn/131.4.1147. [DOI] [PubMed] [Google Scholar]
- 13.DeMarco DM, Santoli D, Zurier RB. Effects of fatty acids on proliferation and activation of human synovial compartment lymphocytes. J Leuk Biol. 1994;56:612–15. doi: 10.1002/jlb.56.5.612. [DOI] [PubMed] [Google Scholar]
- 14.Hosack-Fowler K, Chapkin RS, McMurray DN. Effects of purified dietary n-3 ethyl esters on murine T lymphocyte function. J Immunol. 1993;151:5186–97. [PubMed] [Google Scholar]
- 15.Chow SC, Ansotegui IJ, Jondal M. Inhibition of receptor-mediated calcium influx in T cells by unsaturated non-esterified fatty acids. Biochem J. 1990;267:727–32. doi: 10.1042/bj2670727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stulnig TM, Berger M, Sigmund T, et al. Polyunsaturated fatty acids inhibit T cell signal transduction by modification of detergent-insoluble membrane domains. J Cell Biol. 1998;143:637–44. doi: 10.1083/jcb.143.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arrington JL, Chapkin RS, Switzer KC, et al. Dietary n-3 polyunsaturated fatty acids modulate purified murine T-cell subset activation. Clin Exp Immunol. 2001;125:499–507. doi: 10.1046/j.1365-2249.2001.01627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allison JP. CD28–B7 interactions in T-cell activation. Curr Opin Immunol. 1994;6:414–9. doi: 10.1016/0952-7915(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 19.Chapkin RS, Akoh CC, Miller CC. Influence of dietary n-3 fatty acids on macrophage glycerophospholipid molecular species and peptidoleukotriene synthesis. J Lipid Res. 1991;32:1205–13. [PubMed] [Google Scholar]
- 20.Chapkin RS, Carmichael SL. Effects of dietary n-3 and n-6 polyunsaturated fatty acids on macrophage phospholipid classes and subclasses. Lipids. 1990;25:827–34. doi: 10.1007/BF02535905. [DOI] [PubMed] [Google Scholar]
- 21.Lokesh BR, Hsieh HL, Kinsella JE. Peritoneal macrophages from mice fed dietary (n-3) polyunsaturated fatty acids secrete low levels of prostaglandins. J Nutr. 1986;116:2547–52. doi: 10.1093/jn/116.12.2547. [DOI] [PubMed] [Google Scholar]
- 22.Somers SD, Chapkin RS, Erickson KL. Alteration of in vitro murine peritoneal macrophage function by dietary enrichment of eicosapentaenoic and docosahexaenoic acids in menhaden fish oil. Cell Immunol. 1989;123:201–11. doi: 10.1016/0008-8749(89)90280-3. [DOI] [PubMed] [Google Scholar]
- 23.Hardardottir I, Kinsella JE. Tumor necrosis factor production by murine resident peritoneal macrophages is enhanced by dietary n-3 polyunsaturated fatty acids. Biochem Biophys Acta. 1991;1095:187–95. doi: 10.1016/0167-4889(91)90098-i. [DOI] [PubMed] [Google Scholar]
- 24.Lokesh BR, Sayers TJ, Kinsella JE. Interleukin-1 and tumor necrosis factor synthesis by mouse peritoneal macrophages is enhanced by dietary n-3 polyunsaturated fatty acids. Immunol Lett. 1990;23:281–5. doi: 10.1016/0165-2478(90)90073-y. [DOI] [PubMed] [Google Scholar]
- 25.Crevel RWR, Friend JV, Goodwin BFJ, et al. High-fat diets and the immune response of C57BL mice. Br J Nutr. 1992;67:17–26. doi: 10.1079/bjn19920005. [DOI] [PubMed] [Google Scholar]
- 26.Hubbard NE, Somers SD, Erickson KL. Effect of dietary fish oil on development and selected functions of murine inflammatory macrophages. J Leuk Biol. 1991;49:592–8. doi: 10.1002/jlb.49.6.592. [DOI] [PubMed] [Google Scholar]
- 27.Calder PC, Bond JA, Harvey DJ, et al. Uptake and incorporation of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocytosis. Biochem J. 1990;269:807–14. doi: 10.1042/bj2690807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jolly CA, McMurray DN, Chapkin RS. Effect of dietary n-3 fatty acids on interleukin-2 and interleukin-2 receptor α expression in activated murine lymphocytes. Prostagland Leukotr Essent Fatty Acids. 1998;58:289–93. doi: 10.1016/s0952-3278(98)90038-2. [DOI] [PubMed] [Google Scholar]
- 29.National Research Council. Nutrient Requirements of Laboratory Animals. 4. Washington DC: National Academy Press; 1995. Nutrient requirements of the mouse; pp. 80–102. [Google Scholar]
- 30.Coligan JE, Kruisbeek AM, Margulies DH, et al. Current Protocols in Immunology. New York: Greene Publishing Assoc and Wiley-Interscience; 1991. [Google Scholar]
- 31.Chapkin RS, Somers SD, Erickson KL. Inability of murine peritoneal macrophages to convert linoleic acid into arachidonic acid. Evidence of chain elongation. J Immunol. 1988;140:2350–5. [PubMed] [Google Scholar]
- 32.Kapuscinski J, Darzynkiwicz Z. Spectral properties of fluorchromes used in flow cytometry. Meth Cell Biol. 1990;33:655–69. doi: 10.1016/s0091-679x(08)60559-2. [DOI] [PubMed] [Google Scholar]
- 33.Graybill FA. Theory and Application of the Linear Model. North Scituate: Duxbury Press; 1976. [Google Scholar]
- 34.Jennrich RI, Schluchter MD. Unbalanced repeated-measures models with structured covariance matrices. Biometrics. 1986;42:805–20. [PubMed] [Google Scholar]
- 35.Kuehl RO. Statistical Principles of Research Design and Analysis. Pacific Grove: Duxbury/Thompson Learning; 1994. [Google Scholar]
- 36.Switzer KC, McMurray DN, Chapkin RS. Dietary n-3 polyunsaturated fatty acids selectively promote activation-induced cell death in Th1 cells. Faseb J. 2002;16:A986. [Google Scholar]
- 37.Stulnig TM, Huber J, Leitinger N, et al. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J Biol Chem. 2001;276:37335–40. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- 38.Kane LP, Lin J, Weiss A. Signal transduction by the TCR for antigen. Curr Opin Immunol. 2000;12:242–9. doi: 10.1016/s0952-7915(00)00083-2. [DOI] [PubMed] [Google Scholar]


