Abstract
Follicular lymphomas (FLs) can be difficult to diagnose on aspirated specimens since the architectural pattern is not present. FLs characteristically have rearrangements in the IgH and BCL2 genes resulting from the reciprocal t(14;18) (q32; q21) translocation. Because of the dispersed distribution of breakpoints, fluorescence in situ hybridization (FISH) using genomic probes that span or flank the breakpoints is ideal for detecting this rearrangement in fine-needle aspiration (FNA) biopsies. To develop a set of probes, a bacterial artificial chromosome library was screened and the clones were mapped by fiber FISH. The probes were produced by the direct incorporation of fluorochrome-labeled nucleotides. The colocalization base FISH assay was applied to Cytospin preparations from FNA biopsies of lymph nodes from 26 patients with FL and 10 patients without FL. In those with FL, the percentage of cells with at least one IgH/BCL2 fusion signal ranged from 22% to 100% (mean, 63%), which was statistically significantly higher than that in FL-negative samples (mean, 2.7%). The probes demonstrated a significantly lower cutoff value (7%) in normal controls and effectively reduced the false-positive rate in FL-negative cases. These results were confirmed with fiber FISH assays on the same specimens. This interphase FISH assay is rapid and reliable for detecting rearrangements in the IGH/BCL2 gene, thereby aiding in the diagnosis of FL on FNA biopsy specimens.
Follicular lymphoma (FL) is the most common type of non-Hodgkin’s B-cell lymphoma in the United States. On histology, these lymphomas have a follicular architecture and are composed of a mixture of follicular center cells. Fine-needle aspiration (FNA) of FL can be difficult in some instances because of a lack of architecture and limited immunomarkers. However, since FLs have a characteristic chromosomal abnormality, the t(14;18) translocation, its detection would support the diagnosis of FL.
The t(14;18) translocation leads to the juxtaposition of the BCL2 gene (18q21) and the IgH locus (14q32). This IgH/BCL2 rearrangement results in overexpression of the BCL2 oncogene, which is thought to result in neoplasia by interfering with the normal apoptosis of B lymphocytes. 1 On the BCL2 gene, 60% to 70% of breakpoints are clustered in the major breakpoint region (MBR) located in the 3′ noncoding region, 20% to 30% occur in the minor cluster region (mcr) situated in the 3′ flanking region, and the rest are widely scattered over the BCL2 genomic region. 2 The breakpoints on chromosome 14 mostly lie in the joining region (J) on IgH. 3, 4
Conventional cytogenetic analysis detects the t(14;18) translocation in only about 86% of FLs and requires fresh tissue and meticulous sample preparation. 5 Molecular studies, including Southern blotting and polymerase chain reaction (PCR), fail to detect breakpoints outside the MBR and mcr regions, because of the dispersed breakpoints on both chromosomes. Alternatively, long-distance PCR amplification may detect an IgH/BCL2 rearrangement occurring within the approximately 30-kb region downstream from the MBR of BCL2, but this rather demanding technique is not available in many laboratories. 6
Interphase fluorescence in situ hybridization (FISH) with specific genomic DNA probes for genes not only complements conventional cytogenetic, Southern blot, and PCR methods, it also can detect genomic aberrations at the level of individual cells. Therefore, it is a useful technique for detecting chromosome translocations. Two types of the interphase FISH approach are available for detecting the IgH/BCL2 gene. The first type is a segregation assay with probes for the BCL2 gene that will split in the case of a chromosomal breakpoint. However, the presence of segregation BCL2 signals is not direct proof of the t(14;18) translocation. For example, this type does not differentiate the t(14;18) translocation from polysomy 18 or from the t(2;18) or t(18;22) translocations. 7 The second type is a colocalization base interphase FISH assay that uses specific probes for BCL2 and IgH. The IgH/BCL2 fusion is indicated directly by the touching or superimposed signals of two probes. Because this approach permits rapid screening of interphase nuclei and yields straightforward results, its use is preferable to segregation interphase analysis in clinical samples. However, the low detection efficiency of this second approach has made interpretation of results unreliable because the false-positive and false-negative rates have been high, mainly as a result of previous probe designs and the selection of DNA clones such as yeast artificial chromosome (YAC) and cosmid probes. 8 In addition, the use of these clones requires more complicated DNA preparation methods. Thus, in a diagnostic setting, using these clones as probes severely reduces the practical value of the colocalization base interphase FISH assay. Furthermore, all previous FISH analyses of the IgH/BCL2 rearrangement have been performed on the metaphase and on cytogenetic preparations from peripheral, bone marrow, or tissue biopsy specimens. So far, no data are available on the application of FISH to cytologic specimens such as those obtained by FNA biopsy.
We reasoned that with the correct combination of probes, we should be able to overcome the problems with the colocalization base interphase FISH assay. To detect the IgH/BCL2 rearrangement simply and reliably, we have isolated by PCR and mapped by DNA fiber FISH assay bacterial artificial chromosome (BAC) clones that cover the entire BCL2 gene and the constant (C), J, and diversity (D) regions of the IgH locus. Using these probes, we designed and applied a colocalization base FISH assay to FNA biopsy specimens of lymph nodes from patients with and without FL. We validated the results by PCR analysis and DNA fiber FISH assay with the same probes.
Materials and Methods
Samples
FNA biopsy specimens of lymph nodes from 36 patients showed 26 cases of FL, 8 cases of B-cell small lymphocytic lymphoma (SLL), and 2 cases of large B-cell lymphoma. Four specimens of lymphoid tissue without a diagnosis of lymphoma were used as normal controls (Table 1) . The specimens were analyzed by three-color flow cytometry immunophenotypic studies using a battery of monoclonal antibodies specific for lymphoid antigens. All cases were reviewed by two experienced pathologists (R.L.K. and F.L.), and classified according to the Revised European-American Lymphoma (REAL) classification system. The diagnosis was based on both morphological and immunophenotypic findings. All patients in this study also underwent tissue biopsy to confirm the diagnosis. MBR/IgH and mcr/IgH PCR amplification were also performed in parallel in all FL cases. Peripheral blood leukocytes were obtained from one normal, healthy donor. A human follicular large-cell lymphoma cell line (WSU-NHL) that had the IgH/BCL2 rearrangement was purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany).
Table 1.
Histologic Features of 36 Patients and Results of FISH and PCR Analyses
| Case | Histologic type and grade | Percentage of cells with fusion signals | PCR* |
|---|---|---|---|
| 01 | SLL | 1 | N |
| 02 | SLL | 1 | N |
| 03 | SLL | 2 | − |
| 04 | SLL | 2 | N |
| 05 | SLL | 2 | N |
| 06 | SLL | 3 | − |
| 07 | SLL | 5 | N |
| 08 | SLL | 5 | N |
| 09 | Large B-cell lymphoma | 4 | N |
| 10 | Large B-cell lymphoma | 3 | N |
| 11 | FL(SCL) I | 4 | − |
| 12 | FL(SCL) I | 88 | − |
| 13 | FL(SCL) I | 69 | + |
| 14 | FL(SCL) I | 32 | + |
| 15 | FL(SCL) I | 38 | + |
| 16 | FL(SCL) I | 58 | + |
| 17 | FL(MXL) II | 22 | + |
| 18 | FL(MXL) II | 50 | + |
| 19 | FL(MXL) II | 60 | + |
| 20 | FL(MXL) II | 3 | − |
| 21 | FL(MXL) II | 74 | + |
| 22 | FL(SCL) II | 76 | + |
| 23 | FL(MXL) II | 80 | + |
| 24 | FL(MXL) II | 83.5 | + |
| 25 | FL(MXL) II | 86 | + |
| 26 | FL(MXL) II | 90 | + |
| 27 | FL(MXL) II | 92 | + |
| 28 | FL(LCL) III | 82 | + |
| 29 | FL(LCL) III | 76 | − |
| 30 | FL(LCL) III | 62.5 | + |
| 31 | FL(LCL) III | 48 | + |
| 32 | FL(LCL) III | 48.5 | − |
| 33 | FL(LCL) III | 32 | − |
| 34 | FL(LCL) III | 100 | − |
| 35 | FL(LCL) III | 49 | + |
| 36 | FL(LCL) III | 33 | + |
−, negative; +, positive.
SLL, small lymphocytic lymphoma; follicular lymphoma (small cleaved cell type); FL(MXL), follicular lymphoma (mixed small cleaved cell and large cell type); FL(LCL), follicular lymphoma (large cell type).
Preparation of Interphase Nuclei and DNA Fibers
A portion of the aspirated sample was rinsed in RPMI 1460 (JRH Biosciences, Inc., Lenexa, KS) medium, and the lymphocytes were separated with a Ficoll-Hypaque gradient technique. The mononuclear cells were prepared using cytocentrifugation on silane-coated slides at a concentration of 2 × 106 cells/ml and then fixed in FISH fixative (3 parts methanol:1 part acetic acid).
Normal DNA fiber preparations were made as described previously. 9 In brief, normal peripheral blood lymphocytes, at a concentration of 1 × 107 cells/ml, were embedded in 1% low-melting-point agarose (Promega, Madison, WI). Agarose blocks were incubated at 50°C in 2 mg/ml proteinase K for 48 hours, then washed in three changes of 1X 10 mM Tris-HCl/1 mM EDTA (TE) and treated with 100 μg/ml RNaseA in 2X 37.5 mM NaCl/3.75 mM sodium citrate (SSC) (Vysis, Downers Grove, IL). A small piece of the agarose-embedded DNA was placed on a microscope slide, and 14 μl of water was added. The DNA was extended mechanically on the slide by using the edge of another slide.
DNA fibers from the Cytospin preparations were made as follows: slides were fitted into plastic Cadenza chambers (Thermo Shandon, Inc., Pittsburgh, PA), and 150 μl of lysis solution (5 parts 70 mmol/L NaOH, 2 parts ethanol) was applied to the top of the chamber. The meniscus of the lysis solution was allowed to move down the slide. As the level droped below the frosted edge of the slide, 200 μl of ethanol was added and allowed to drain briefly. The slides were then fixed in Carnoy’s solution for 10 minutes.
Probes for FISH Assays
To obtain an appropriate BCL2 probe for FISH assay, we screened an 8X genomic-coverage BAC library (Research Genetics Inc., Huntsville, AL) with a PCR-based screening protocol with specific primers for exon II and mcr. 10 The size of each BAC was estimated from agarose gels after enzyme digestion. The chromosomal location of the clones was confirmed on a normal metaphase spread in combination with a centromeric probe for chromosome 18 (CEP 18; Vysis, Downers Grove, IL). Mapping of the overlapping region of the BACs was performed on normal DNA fibers with dual-color labeling. 11
Similarly, a BAC clone for IgH was isolated by PCR with human STS SHGC-11760 primer, and clone mapping was performed with specific primers for the C and J regions. 11, 12, 13 The chromosomal location was confirmed using a normal metaphase spread in combination with a centromeric probe for chromosome 14 (CEP 14; Vysis). The size and mapping region of the BAC probe on IgH were analyzed on normal DNA fibers with a known 110-kb BAC clone (kindly provided by Mariano Rocchi, DAPEG Sezione di Genetica, Bari, Italy), which was mapped on the J and D regions.
FISH Assays
For FISH assays, the IgH probe was directly labeled by nick translation with SpectrumGreen-dUTP (Vysis), and the BCL2 probe was labeled with SpectrumOrange-dUTP (Vysis). One hundred nanograms of each probe was mixed with a 30-fold excess of human Cot-1 DNA (BRL-Life Technologies, Rockville, MD) in 10 μl of LSI hybridization buffer (Vysis), and mounted on a slide. Hybridization was performed using the HYBrite hybridization system (Vysis), and post-washing was performed as described previously. 14 Interphase nuclei and DNA fibers were carefully examined using Leica microscopes equipped with appropriate filter sets for visualizing SpectrumGreen, SpectrumOrange, and DAPI counterstains (Leica Microsystems, Inc., Buffalo, NY). Images were captured using CytoVision (Applied Imaging Corporation, Santa Clara, CA).
PCR Analysis
PCR analysis was carried out to determine the presence of JH/BCL2 fusion DNA sequences consistent with the t(14;18) (q32;q21) translocation using the primers and methods described by Lee et al. 15, 16
Results
Validation of Probes
One BAC clone (442F20) was mapped by PCR on the C and J regions of IgH, spanning 220 kb. The size and mapping region of the probe was confirmed by dual-color FISH on normal DNA fiber with the known 110-kb BAC dJ998D24. The 442F20 clone overlaps with dJ998D24 with a 20-kb common region. Therefore, the IgH probe is 310 kb long, covering the C, J, and D regions of the IgH locus (Figure 1) .
Figure 1.
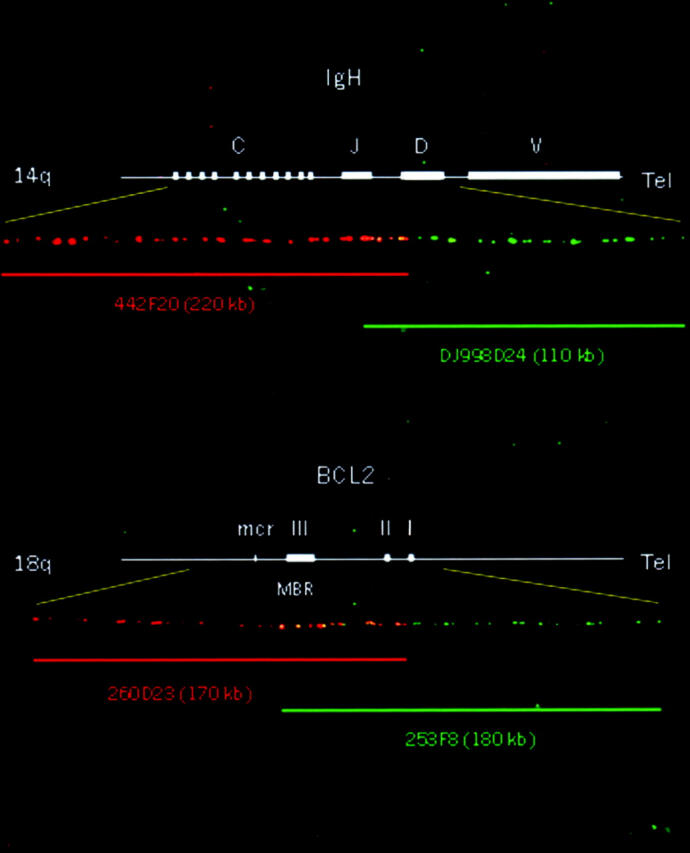
Mapping of IgH and BCL2 probes by fiber FISH assay on DNA fibers of normal peripheral blood leukocytes. The top fiber shows the position of bacterial artificial chromosomes 442F20 (orange bar) and dJ998D24 (green bar) relative to the IgH locus. 442F20 was mapped on the constant and joining regions of IgH; it spans 220 kb and overlaps with the 110-kb dJ998D24, which covers the joining and diversity regions. The overlapping region is 20 kb long, indicated by the yellow barcode. The total size of the IgH probe is 310 kb. The bottom fiber shows the position of BACs 260D23 (orange bar) and 253F8 (green bar) relative to the BCL2 gene. 260D23 was mapped on exons II and III and on the major breakpoint and minor cluster regions, spanning 170 kb. 253F8 is 180 kb long, covering exons I, II, and III. The clones are connected to each other with approximately 50 kb of overlap (yellow). The total length of the BCL2 probe is 300 kb, spanning the entire 225 kb of BCL2 and its 5′ and 3′ flanking genomic regions.
Two positive BAC clones were identified for the BCL2 gene: 253F8 was positive for the primer of exon II, and 260D23 was positive for the MBR and the mcr. These clones were determined to be approximately 180 kb and 170 kb long, respectively, by agarose gel. They overlap approximately 50 kb on normal DNA fibers (Figure 1) . The total length of the BCL2 probe was estimated to be >300 kb, spanning the entire 225 kb of BCL2 and its 5′ and 3′ flanking genomic regions. A FISH assay on the normal metaphase confirmed the chromosomal location of the IgH probe on 14q32 and the signals of the BCL2 probe on 18q21 (Figure 2A) .
Figure 2.
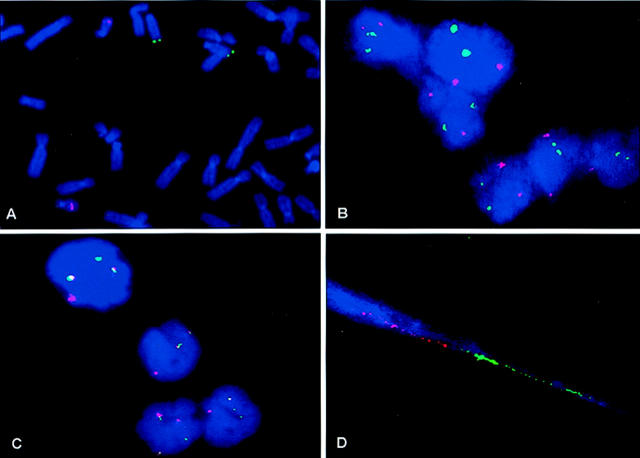
A: FISH on normal metaphase shows the chromosomal locations of the IgH probe on 14q32 (green) and the BCL2 probe on 18q21 (orange). B: Normal interphase nuclei show two IgH probe signals (green) and two BCL2 probe signals (orange). C: Detection of t(14;18) (14q32;18q21) in interphase nuclei of a specimen from a patient with follicular lymphoma shows yellow or touching signals of two different color probes, indicating colocalization of IgH/BCL2 fusion. D: Detection of t(14;18) (14q32;18q21) with fiber FISH on a specimen from a patient with follicular lymphoma; a single linear DNA fiber with two juxtaposed color barcode signals indicates IgH (green) and BCL2 (orange) fusion.
On the control interphase from the normal lymphoid tissues, two green signals (corresponding to the IgH probe) and two orange signals (corresponding to the BCL2 probe) were detected (Figure 2B) . Colocalization of the two probes or IGH/BCL2 fusion (Figure 2C) was considered present by interphase FISH if there were only yellow signals or two touching signals without any interval, whereas with DNA fiber FISH, a single linear DNA molecule with two juxtaposed color barcode signals was considered positive for IgH/BCL2 fusion (Figure 2D) .
For normal controls, we analyzed 200 consecutive nuclei per slide from the four lymph nodes without malignancy. Colocalized fusion signals were found in 0% to 5% of the cells (mean false positivity, 2%), whereas the positive IgH/BCL2 rearrangement cell line (WSU-NHL) showed that 76% of cells had at least one fusion signal. The cutoff value for positivity was determined to be 7% (mean ± 3 standard deviations). The same normal control specimens were analyzed by fiber FISH. At least 30 fibers were scored for the presence of the IgH/BCL2 rearrangement. Juxtaposition signals ranged from 0% to 3%. The cutoff value for positivity was fixed at 7%.
Detection of IgH/BCL2 Rearrangement by FISH in Patients
The IgH/BCL2 rearrangement was found in 24 of the 26 patients with FL (Table 1) . In the positive samples, the percentage of cells with at least one IgH/BCL2 fusion was variable, ranging from 22% to 100% (mean, 63%), statistically significantly higher than the 7% cutoff value. Although the percentage of cells with fusion of two probes in the FL-negative samples ranged from 1% to 5%, this range was significantly lower than that observed for FL-positive cases; however, no significant difference occurred between this range and that of the normal controls (Table 1) .
In all 24 positive cases detected by interphase FISH, the IgH/BCL2 rearrangement was confirmed by fiber FISH with the same probes, and the abnormality occurred in 10% to 79%. The two cases that were negative for the rearrangement by interphase FISH were also negative by fiber FISH, although 36% of the cells in one of the two cases showed two IgH signals and multiple BCL2 signals. A dual-color FISH analysis of the case with the CEP 18 and BCL2 BAC probes always showed two signals of the CEP 18 per cell, which excludes the possibility that multiple BCL2 signals were simply a result of aneuploidy of the entire chromosome 18 in the cases.
Comparison of FISH Assay and PCR Analyses
Among the 24 FL-positive cases detected by both FISH techniques, 19 were also positive by PCR analysis. The two cases that were negative by the FISH techniques also were negative by PCR. Only two of the FL-negative samples were tested by PCR, and they were negative for the IgH/BCL2 rearrangement (Table 1) .
Discussion
Although typical cases of FL are easily recognized with morphological and immunological analyses, the diagnosis may be difficult in atypical cases or with limited sample. Therefore, a reliable test that could aid in the diagnosis of FLs on small samples such as those obtained from FNA biopsies would be clinically useful.
Our new set of DNA probes rapidly and reliably detected the IgH/BCL2 rearrangement in 92% of the specimens from patients with FL without false positivity in the normal controls or the FL-negative specimens. This 92% positivity demonstrated a high level of diagnostic sensitivity of this set of probes in cytologic preparations from FL; the sensitivity of the probes in interphase was confirmed by fiber FISH. Our results suggest that these new probes are particularly advantageous for use with cytologic specimens that have limited cellularity and contain desegregated cells.
The colocalization base interphase FISH approach was previously reported to have a low detection efficiency because of highly variable false-positive rates in normal nuclei and false-negative rate in positive cells. 17 It is extremely difficult to distinguish positive cases from normal samples when the percentage of cells with fusion signals is close to the cutoff value, particularly in tumor specimens that have a large number of normal cells. The false-positive rate may be ascribed mainly to unstable and chimeric YAC clone DNA because the YAC clones contain unstable and chimeric DNA sequences, which often yield diffuse, nonspecific signals. 18, 19, 20 In addition, detection of the YAC probe requires an antibody immunostaining technique; this strategy also increases nonspecific signals. Therefore, YAC signals are scored ambiguously and the rates of false fusion and coincidental colocalization signals are high, further yielding a high average rate of false-positive nuclei, especially in the interphase. 21 On the other hand, the false-negative rate may frequently result from probes that consist of a pool of YAC and a small clone, such as a phage or cosmid, because these clones cover only partial regions of genes and have genomic gaps. In this case, some complex rearrangements, such as excision of the BCL2 gene from chromosome 18 and insertion into the IgH locus and breakpoints that locate very telomerically on one gene and very centromerically on another gene, would be undetected by one 5′ probe and one 3′ probe of BCL2 or IgH. 22 This is especially true when the cells are in the S/G2 phases of the cell cycle. Furthermore, the physical distance of the two probes within fusion signals may vary widely as the sizes of the cells and nuclei and the location of the breakpoints change. Therefore, if there are gaps in both probes, it will be very difficult to fix a cutoff distance for determining whether signals are fused or separated. This would lead to high inter-observer variability making the interpretation of the results even more difficult. 4, 17, 23
We set out to develop new sets of probes by using BAC clones. The insert of the BAC clone has high genomic coverage and is 80 to 300 kb long, which is several times longer than that in a cosmid or phage (10 to 30 kb), and therefore provides relatively efficient analysis data. 24 We labeled the DNA directly without amplifying human DNA using Alu-PCR because the BAC clone contains a highly stable and rich human genomic insert. This set of BAC clones always results in clear, sharp, bright spots on monolayer cytologic preparations. In addition, the probes were produced by the direct incorporation of fluorochrome-labeled nucleotides that eliminate the need for immunostaining to amplify the signals, thereby reducing nonspecific hybridization. In normal controls, the probes have a low cutoff value and show a significantly low percentage of false-positive IgH/BCL2 fusion cells in FL-negative samples, effectively eliminating false positivity.
As shown with normal DNA fibers, two BAC clones of the BCL2 probe have a minimal overlap of 50 kb, covering the entire gene from upstream of 3′ to downstream of 5′. The IgH BAC probe spans the C, J, and D regions of the locus. There are no sequence gaps in the probes, effectively eliminating the possibility of false negatives. Because breakpoints always occur in the J region of IgH in FL whenever t(14;18) involves the IgH/BCL2 rearrangement, breakage of the BCL2 is demonstrated by fusion of the different labeled probes on chromosome 14,18 or both. In this study, the probes were designed to eliminate the false-positive rate. Only the touching or overlapping of two different color probes was considered to be a fusion signal, and this was confirmed by the close connection of two different color barcode signals in a single DNA fiber on the same cases. According to this criterion, an elevated percentage of nuclei with at least one fusion signal was demonstrated in 24 of the 26 FL cases (mean, 63%). In contrast, no FL-negative sample contained more than 5% of nuclei with fusion signals. This difference in the percentage of fusion signals between FL-positive and FL-negative cases was statistically significant.
PCR analysis detected the IgH/BCL2 rearrangement in 19 of 26 cases of FL. Even though it can confirm a cytologic diagnosis of lymphoma in FNA specimens, PCR is of limited value in the diagnosis of FL without morphological or clinical support because of its high rate of false negativity. 25 Compared with PCR analysis, the dual-color colocalization base FISH assay with our set of probes is much more sensitive (92 vs. 73%) for detecting IgH/BCL2 rearrangement in cytologic specimens.
Two documented FL-positive cases were negative for IgH/BCL2 fusion by both FISH and PCR analyses. It is interesting to note, however, that 36% of cells in one of the two samples demonstrated multiple BCL2 signals by FISH, suggesting a split, amplification, or rearrangement of the BCL2 gene and resulting in overexpression of the BCL2 oncogene. This probably indicates that the transformation of FL cells in this case was not associated with a chromosomal translocation involving IgH but instead was associated with another chromosomal locus or mechanism, such as mutations of the BCL2 gene. This might cause increased expression of BCL2 by affecting the interactions of BCL2 with other proteins. 26, 27, 28 However, with our unique probes, we could still detect the genomic rearrangement of the BCL2 gene.
Recently, using a commercial set of probes (Vysis), Frater et al 29 detected t(14; 18) (q32; q21) translocation in 25 of 39 FL cases. Their sensitivity (64%) is the lowest in all reported studies using FISH to detect t(14;18) and is even lower than that in the studies using PCR and conventional cytogenetic methods. 4, 8, 21, 23, 29 The practical value of the commercial probes needs to be validated in further well-designed studies, especially in terms of sensitivity, specificity, and cutoff value. Our new set of probes is more convenient and more sensitive than those previously used to detect the IgH/BCL2 fusion. Our colocalization base FISH assay can be a useful adjunct in diagnosing FLs, especially on small samples such as those obtained by FNA biopsy. Thus, these small samples can be accurately classified which will aid in predicting the biological and clinical behavior of the disease.
Acknowledgments
We thank Ms. Vicki L. Hopwood for help with the fiber FISH imaging analysis and Ms. Mariann Crapanzano and Ms. Karen F. Philips for critically reading the manuscript.
Address reprint requests to Dr. Ruth L. Katz, Department of Pathology, Box 53, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX. E-mail: rkatz@mail.mdanderson.org.
Footnotes
Supported by National Institutes of Health grant RFP N01 CN 85083 57 (to R.L.K), Developmental Project/Career Development Award from the University of Texas Specialized Programs of Research Excellence (SPORE) in Lung Cancer (P50 CA70907) (to F.J.), and the W. M. Keck Center for Cancer Gene Therapy Award (to F.J.).
References
- 1.Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ: Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990, 348:334-336 [DOI] [PubMed] [Google Scholar]
- 2.Buchonnet G, Lenain P, Ruminy P, Lepretre S, Stamatoullas A, Parmentier F, Jardin F, Duval C, Tilly H, Bastard C: Characterization of BCL2-JH rearrangements in follicular lymphoma: PCR detection of 3′ BCL2 breakpoints and evidence of a new cluster. Leukemia 2000, 14:1563-1569 [DOI] [PubMed] [Google Scholar]
- 3.Yunis JJ, Frizzera G, Oken MM, McKenna J, Theologides A, Arnesen M: Multiple recurrent genomic defects in follicular lymphoma: a possible model for cancer. N Engl J Med 1987, 316:79-84 [DOI] [PubMed] [Google Scholar]
- 4.Vaandrager JW, Schuuring E, Raap T, Philippo K, Kleiverda K, Kluin P: Interphase FISH detection of BCL2 rearrangement in follicular lymphoma using breakpoint-flanking probes. Genes Chromosomes Cancer 2000, 27:85-94 [PubMed] [Google Scholar]
- 5.Poetsch M, Weber-Matthiesen K, Plendl HJ, Grote W, Schlegelberger B: Detection of the t(14;18) chromosomal translocation by interphase cytogenetics with yeast-artificial-chromosome probes in follicular lymphoma and non-neoplastic lymphoproliferation. J Clin Oncol 1996, 14:963-969 [DOI] [PubMed] [Google Scholar]
- 6.Akasaka T, Muramatsu M, Ohno H, Miura I, Tatsumi E, Fukuhara S, Mori T, Okuma M: Application of long-distance polymerase chain reaction to detection of junctional sequences created by chromosomal translocation in mature B-cell neoplasms. Blood 1996, 88:985-994 [PubMed] [Google Scholar]
- 7.Bertheas MF, Bachy M, Magaud JP, Rimokh R, Vasselon C, Berger F, Oriol PC, Jaubert J, Reynaud J, Brizard CP: T(2;18) and t(18;22) variant chromosomal translocations in B-cell malignancies. Leuk Lymphoma 1992, 8:197-200 [DOI] [PubMed] [Google Scholar]
- 8.von Bergh A, Emanuel B, van Zelderen-Bhola S, Smetsers T, van Soest R, Stul M, Vranckx H, Schuuring E, Hagemeijer A, Kluin P: A DNA probe combination for improved detection of MLL/11q23 breakpoints by double-color interphase-FISH in acute leukemias. Genes Chromosomes Cancer 2000, 28:14-22 [DOI] [PubMed] [Google Scholar]
- 9.Heiskanen M, Karhu R, Hellsten E, Peltonen L, Kallioniemi OP, Palotie A.: High resolution mapping using fluorescence in situ hybridization to extended DNA fibers prepared from agarose-embedded cells. Biotechniques 1994, 17:928-932 [PubMed] [Google Scholar]
- 10.Silverman GA, Jockel JI, Domer PH, Mohr RM, Taillon-Miller P, Korsmeyer SJ: Yeast artificial chromosome cloning of a two-megabase-size contig within chromosomal band 18q21 establishes physical linkage between BCL2 and plasminogen activator inhibitor type-2. Genomics 1991, 9:219-228 [DOI] [PubMed] [Google Scholar]
- 11.Heiskanen M, Hellsten E, Kallioniemi OP, Makela TP, Alitalo K, Peltonen L, Palotie A: Visual mapping by fiber-FISH. Genomics 1995, 30:31-36 [DOI] [PubMed] [Google Scholar]
- 12.Bergsagel PL, Chesi M, Nardini E, Brents LA, Kirby SL, Kuehl WM: Promiscuous translocations into immunoglobulin heavy chain switch regions in multiple myeloma. Proc Natl Acad Sci USA 1996, 93:13931-13936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssen JW, Vaandrager JW, Heuser T, Jauch A, Kluin PM, Geelen E, Bergsagel PL, Kuehl WM, Drexler HG, Otsuki T, Bartram CR, Schuuring E: Concurrent activation of a novel putative transforming gene, myeov, and cyclin D1 in a subset of multiple myeloma cell lines with t(11;14)(q13;q32). Blood 2000, 15:2691-2698 [PubMed] [Google Scholar]
- 14.Katz RL, Caraway NP, Gu J, Jiang F, Pasco-Miller LA, Glassman AB, Luthra R, Hayes KJ, Romaguera JE, Cabanillas FF, Medeiros LJ: Detection of chromosome 11q13 breakpoints by interphase fluorescence in situ hybridization: a useful ancillary method for the diagnosis of mantle- cell lymphoma. Am J Clin Pathol 2000, 114:248-257 [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Guillermo A, Cabanillas F, McDonnell TI, McLaughlin P, Smith T, Pugh W, Hagemeister F, Rodriguez MA, Romaguera JE, Younes A, Sarris AH, Preti HA, Lee MS: Correlation of bcl-2 rearrangement with clinical characteristics and outcome in indolent follicular lymphoma. Blood 1999, 93:3081-3087 [PubMed] [Google Scholar]
- 16.Soubeyran P, Cabanillas F, Lee MS: Analysis of the expression of the hybrid gene bcl-2/IgH in follicular lymphomas. Blood 1993, 81:122-127 [PubMed] [Google Scholar]
- 17.Chase A, Grand F, Zhang JG, Blackett N, Goldman J, Gordon M: Factors influencing the false positive and negative rates of BCR-ABL fluorescence in situ hybridization. Genes Chromosomes Cancer 1997, 18:246-253 [PubMed] [Google Scholar]
- 18.Lengauer C, Speicher MR, Cremer T: FISH of Alu-PCR-amplified YAC clones and applications in tumor cytogenetics. Methods Mol Biol 1994, 33:85-94 [DOI] [PubMed] [Google Scholar]
- 19.Gosden J, Breen M, Lawson D: Alu- and L1-primed PCR-generated probes for non-isotopic in situ hybridization. Methods Mol Biol 1994, 29:479-492 [DOI] [PubMed] [Google Scholar]
- 20.Shibasaki Y, Maule JC, Devon RS, Slorach EM, Gosden JR, Porteous DJ, Brookes AJ: Catch-linker + PCR labeling: a simple method to generate fluorescence in situ hybridization probes from yeast artificial chromosomes. PCR Methods and Applications 1995, 4:209-211 [DOI] [PubMed] [Google Scholar]
- 21.Taniwaki M, Sliverman GA, Nishida K, Horiike S, Misawa S, Shimazaki C, Miura I, Nagai M, Abe M, Fukuhara S: Translocations and amplification of the BCL2 gene are detected in interphase nuclei of non-Hodgkin’s lymphoma by in situ hybridization with yeast artificial chromosome clones. Blood 1995, 86:1481-1486 [PubMed] [Google Scholar]
- 22.Vaandrager JW, Schuuring E, Philippo K, Kluin PM: V(D)J recombinase-mediated transposition of the BCL2 gene to the IGH locus in follicular lymphoma. Blood 2000, 96:1947-1952 [PubMed] [Google Scholar]
- 23.Rack KA, Salomon-Nguyen F, Radford-Weiss I, Gil MO, Schmitt C, Belanger C, Nusbaum S, Vekemans M, Valensi F, Macintyre EA: FISH detection of chromosome 14q32/IgH translocations: evaluation in follicular lymphoma. Br J Haematol 1998, 103:495-504 [DOI] [PubMed] [Google Scholar]
- 24.Shizuya H, Kouros-Mehr H: The development and applications of the bacterial artificial chromosome cloning system. Keio J Med 2001, 50:26-30 [DOI] [PubMed] [Google Scholar]
- 25.Aiello A, Delia D, Giardini R, Alasio L, Bartoli C, Pierotti MA, Pilotti S: PCR analysis of IgH and BCL2 gene rearrangement in the diagnosis of follicular lymphoma in lymph node fine-needle aspiration: a critical appraisal. Diagn Mol Pathol 1997, 6:154-160 [DOI] [PubMed] [Google Scholar]
- 26.Lambrechts AC, Looijenga LH, van’t Veer MB, van Echten J, Timens W, Oosterhuis JW: Lymphomas with testicular localisation show a consistent BCL-2 expression without a translocation (14;18): a molecular and immunohistochemical study. Br J Cancer 1995, 71:73-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matolcsy A, Casali P, Warnke RA, Knowles DM: Morphologic transformation of follicular lymphoma is associated with somatic mutation of the translocated Bcl-2 gene. Blood 1996, 88:3937-3944 [PubMed] [Google Scholar]
- 28.Monni O, Franssila K, Joensuu H, Knuutila S: BCL2 overexpression in diffuse large B-cell lymphoma. Leuk Lymphoma 1999, 34:45-52 [DOI] [PubMed] [Google Scholar]
- 29.Frater JL, Tsiftsakis EK, Hsi ED, Pettay J, Tubbs RR: Use of novel t(11;14) and t(14;18) dual-fusion fluorescence in situ hybridization probes in the differential diagnosis of lymphomas of small lymphocytes. Diagn Mol Pathol 2001, 10:214-222 [DOI] [PubMed] [Google Scholar]