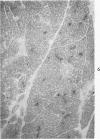
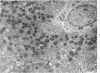
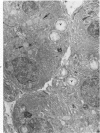
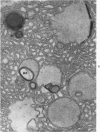
Full text
PDF









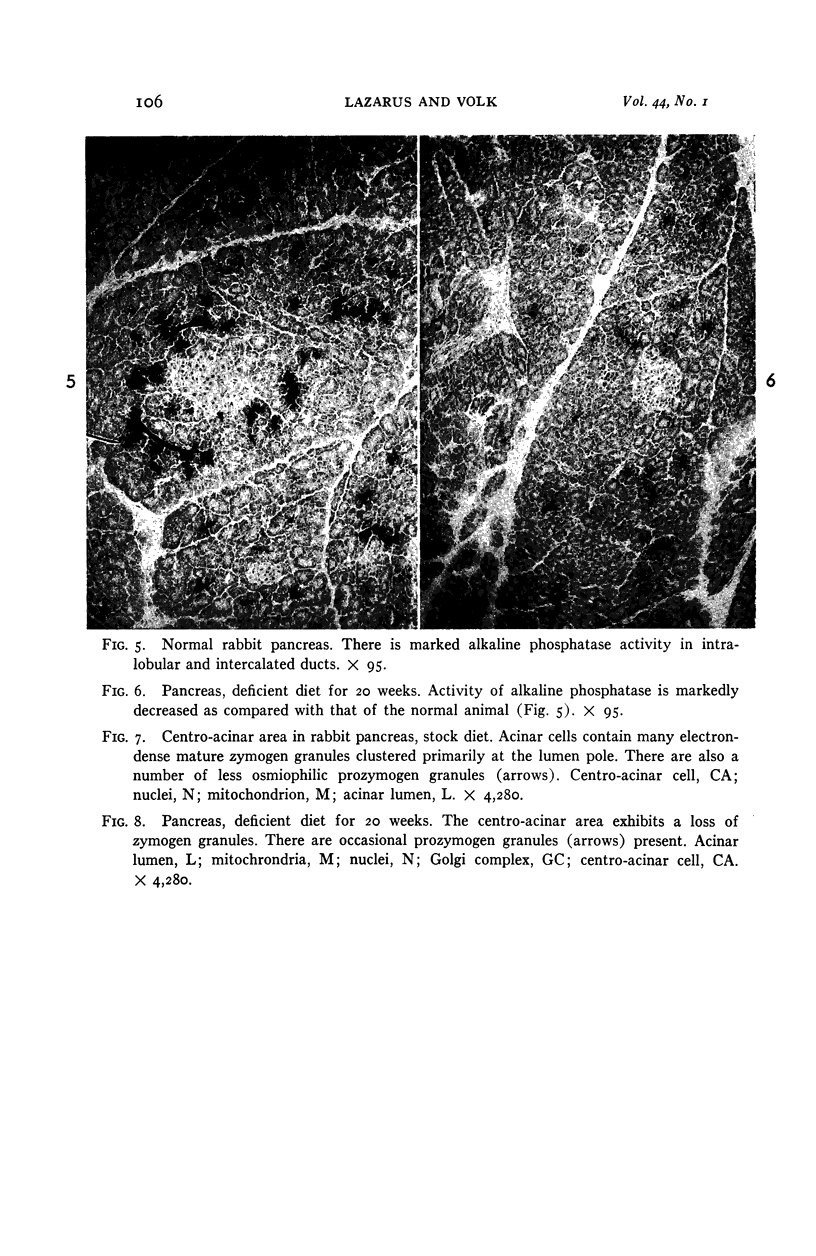
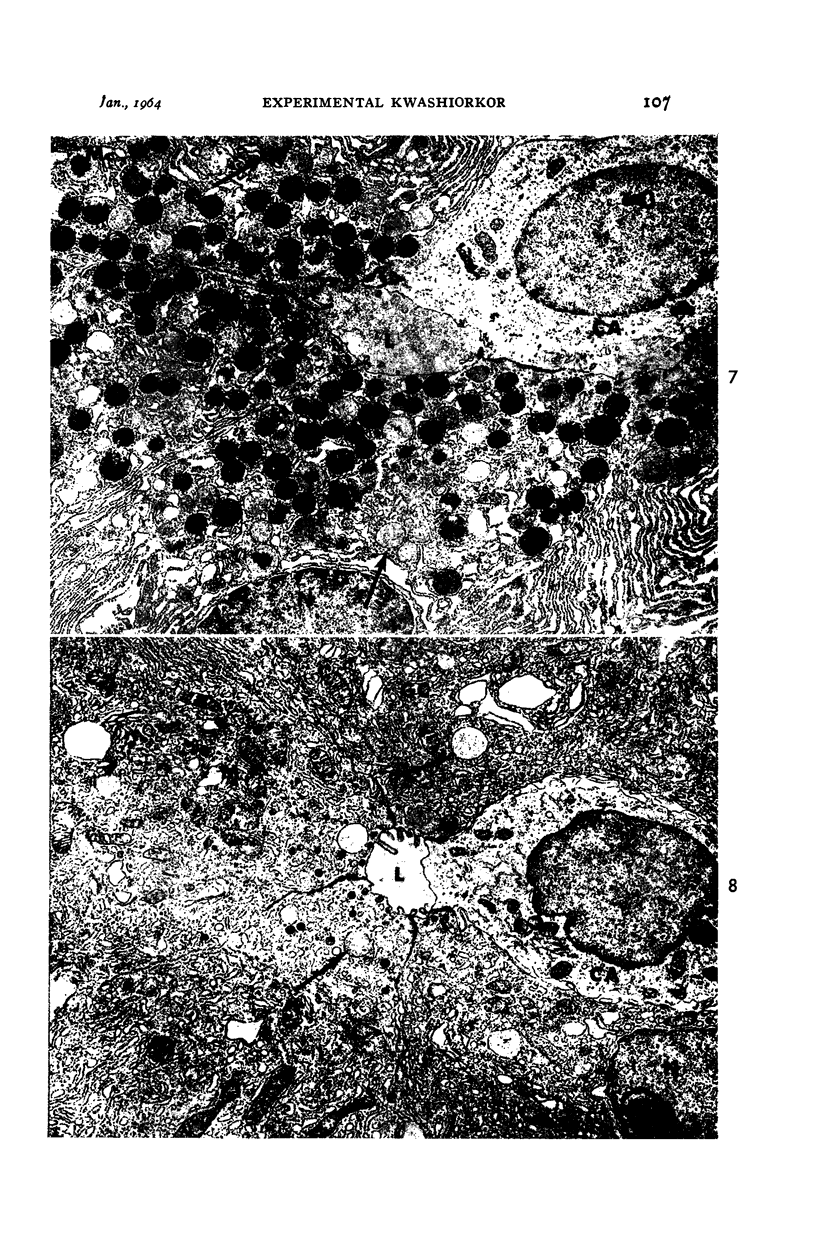
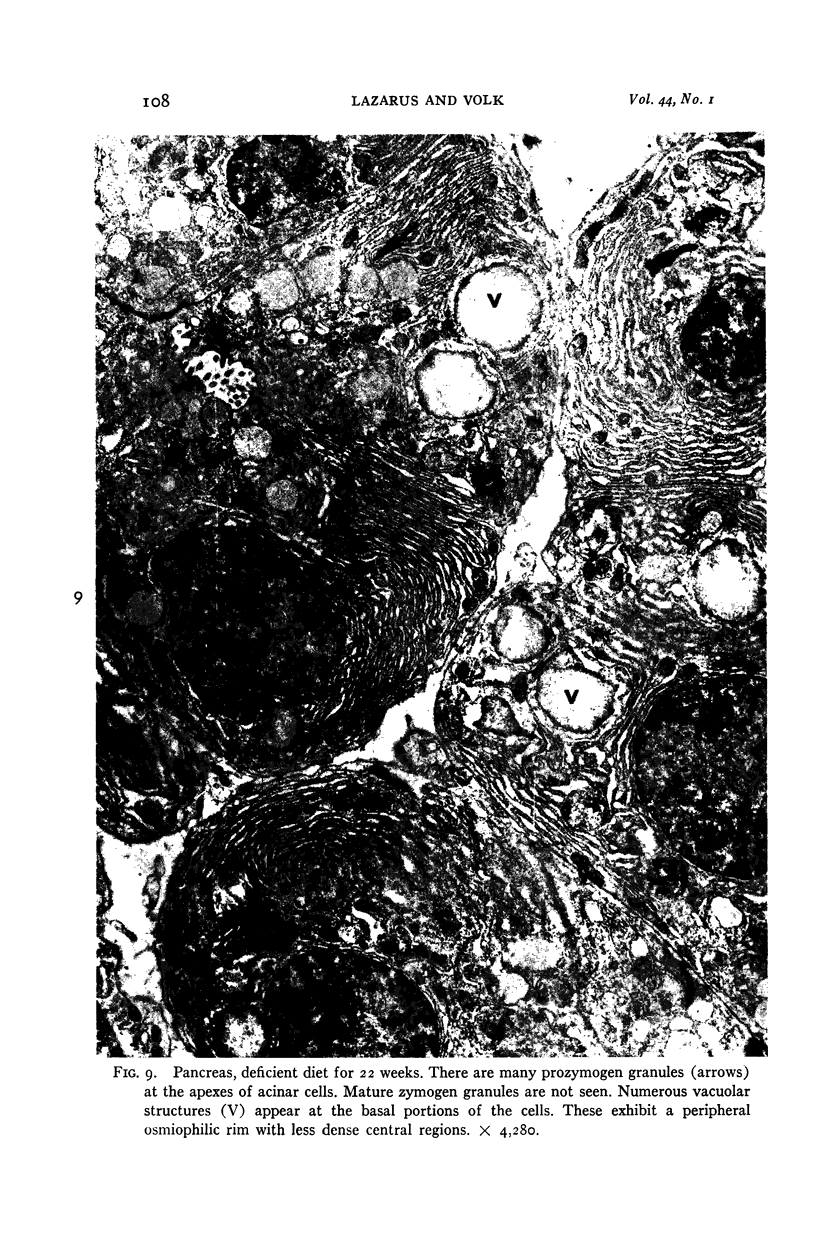
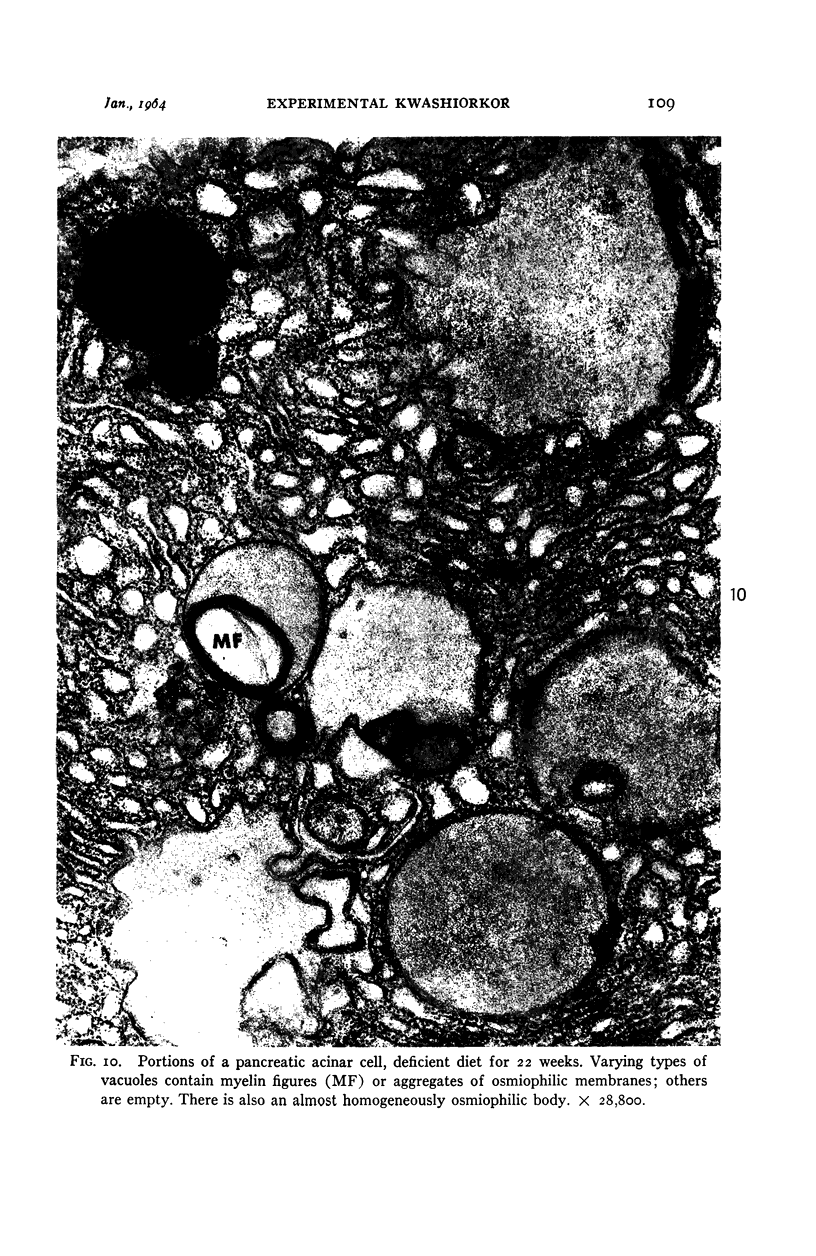
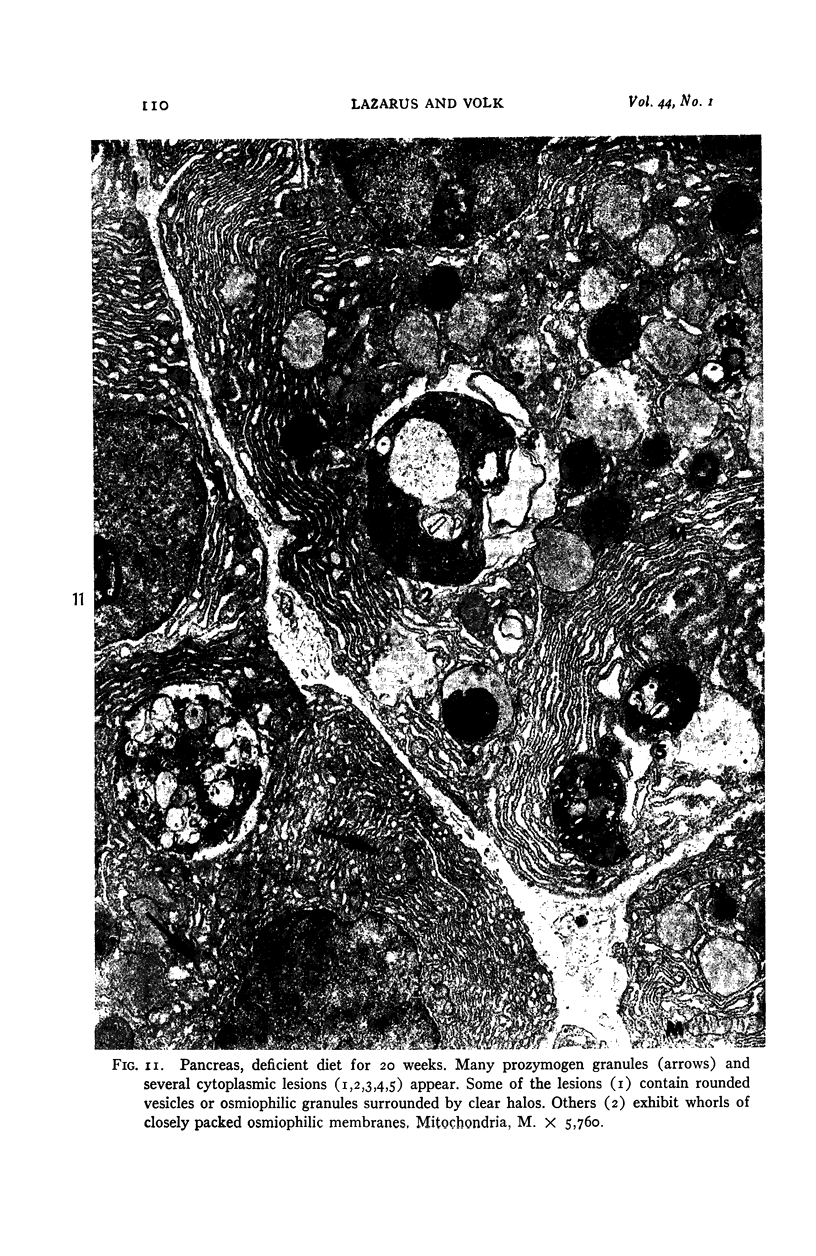
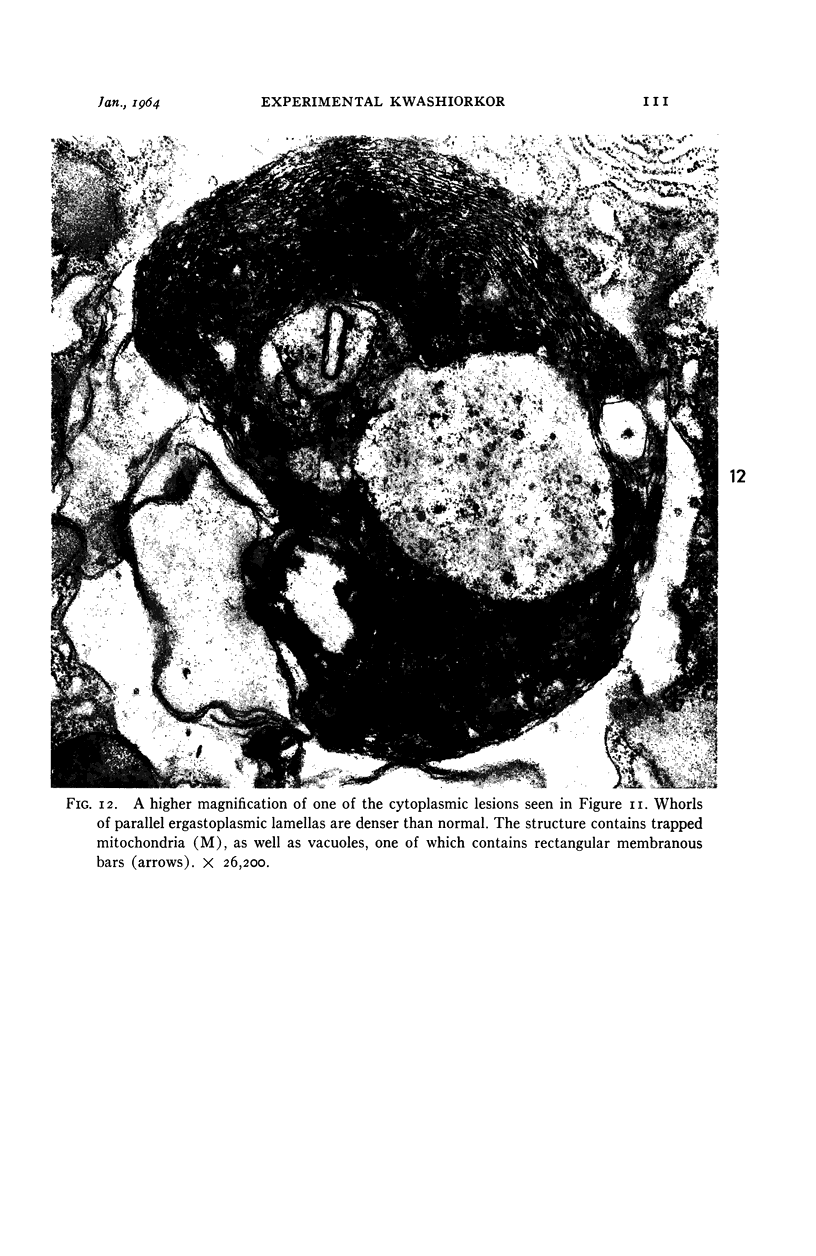
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS C. W., FERNAND V. S., SCHNIEDEN H. Histochemistry of a condition resembling kwashiorkor produced in rodents by a low protein-high carbohydrate diet (Cassava). Br J Exp Pathol. 1958 Aug;39(4):393–404. [PMC free article] [PubMed] [Google Scholar]
- ASHFORD T. P., PORTER K. R. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol. 1962 Jan;12:198–202. doi: 10.1083/jcb.12.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENCOSME S. A., STONE R. S., LATTA H., MADDEN S. C. Acute reactions with collagen production in renal glomeruli of rats as studied electron microscopically. J Ultrastruct Res. 1959 Dec;3:171–185. doi: 10.1016/s0022-5320(59)90013-9. [DOI] [PubMed] [Google Scholar]
- BERNHARD W., ROUILLER C. Microbodies and the problem of mitochondrial regeneration in liver cells. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):355–360. doi: 10.1083/jcb.2.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BINGGELI M. F. Abnormal intranuclear and cytoplasmic formations associated with a chemically induced, transplantable chicken sarcoma. J Biophys Biochem Cytol. 1959 Jan 25;5(1):143–152. doi: 10.1083/jcb.5.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAULFIELD J. B. Effects of varying the vehicle for OsO4 in tissue fixation. J Biophys Biochem Cytol. 1957 Sep 25;3(5):827–830. doi: 10.1083/jcb.3.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAEMS W. T., van RIJSSEL T. The fine structure of the peribiliary dense bodies in mouse liver tissue. J Ultrastruct Res. 1961 Jun;5:263–290. doi: 10.1016/s0022-5320(61)90020-x. [DOI] [PubMed] [Google Scholar]
- ESSNER E., NOVIKOFF A. B. Human hepatocellular pigments and lysosomes. J Ultrastruct Res. 1960 Jun;3:374–391. doi: 10.1016/s0022-5320(60)90016-2. [DOI] [PubMed] [Google Scholar]
- FARBER E., POPPER H. Production of acute pancreatitis with ethionine and its prevention by methionine. Proc Soc Exp Biol Med. 1950 Aug;74(4):838–840. doi: 10.3181/00379727-74-18062. [DOI] [PubMed] [Google Scholar]
- FARQUHAR M. G., WELLINGS S. R. Electron microscopic evidence suggesting secretory granule formation within the Golgi apparatus. J Biophys Biochem Cytol. 1957 Mar 25;3(2):319–322. doi: 10.1083/jcb.3.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERREIRA D. L'ultrastructure des cellules du pancréas endocrine chez l'embryon et le rat nouveau-né. J Ultrastruct Res. 1957 Nov;1(1):14–25. doi: 10.1016/s0022-5320(57)80009-4. [DOI] [PubMed] [Google Scholar]
- FITZGERALD P. J., ALVIZOURI M. Rapid restitution of the rat pancreas following acinar cell necrosis subsequent to ethionine. Nature. 1952 Nov 29;170(4335):929–930. doi: 10.1038/170929b0. [DOI] [PubMed] [Google Scholar]
- GOLDBERG R. C., CHAIKOFF I. L. Selective pancreatic acinar destruction by diethionine. AMA Arch Pathol. 1951 Sep;52(3):230–238. [PubMed] [Google Scholar]
- HERMAN L., FITZGERALD P. J. Restitution of pancreatic acinar cells following ethionine. J Cell Biol. 1962 Feb;12:297–312. doi: 10.1083/jcb.12.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERMAN L., FITZGERALD P. J. The degenerative changes in pancreatic acinar cells caused by DL-ethionine. J Cell Biol. 1962 Feb;12:277–296. doi: 10.1083/jcb.12.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESS R., SCARPELLI D. G., PEARSE A. G. The cytochemical localization of oxidative enzymes. II. Pyridine nucleotide-linked dehydrogenases. J Biophys Biochem Cytol. 1958 Nov 25;4(6):753–760. doi: 10.1083/jcb.4.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HRUBAN Z., SWIFT H., WISSLER R. W. Analog-induced inclusions in pancreatic acinar cells. J Ultrastruct Res. 1962 Oct;7:273–285. doi: 10.1016/s0022-5320(62)90023-0. [DOI] [PubMed] [Google Scholar]
- LAZARUS S. S. A combined periodic acid-Schiff trichrome stain. AMA Arch Pathol. 1958 Dec;66(6):767–772. [PubMed] [Google Scholar]
- LAZARUS S. S. Acid and glucose-6-phosphatase activity of pancreatic B cells after cortisone and sulfonylureas. Proc Soc Exp Biol Med. 1959 Nov;102:303–306. doi: 10.3181/00379727-102-25227. [DOI] [PubMed] [Google Scholar]
- LAZARUS S. S., BARDEN H., BRADSHAW M. Pancreatic beta cells and alloxan toxicity. Enzymatic histochemistry and toxic mechanism. Arch Pathol. 1962 Mar;73:210–222. [PubMed] [Google Scholar]
- LAZARUS S. S., BRADSHAW M. Oxidative pathways in pancreatic B cells. Proc Soc Exp Biol Med. 1959 Nov;102:463–468. doi: 10.3181/00379727-102-25286. [DOI] [PubMed] [Google Scholar]
- LAZARUS S. S. Demonstration of glucose-6-phosphatase in mammalian pancreas. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:819–822. doi: 10.3181/00379727-101-25108. [DOI] [PubMed] [Google Scholar]
- LINDNER E. Die submikroskopische Morphologie des Herzmuskels. Z Zellforsch Mikrosk Anat. 1957;45(6):702–746. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOE H., BEHNKE O. Cytoplasmic bodies containing mitochondria, ribosomes, and rough surfaced endoplasmic membranes in the epithelium of the small intestine of newborn rats. J Cell Biol. 1962 Apr;13:168–171. doi: 10.1083/jcb.13.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLNAR Z., LARSEN K., SPARGO B. Cardiac changes in the potassium-depleted rat. Arch Pathol. 1962 Oct;74:339–347. [PubMed] [Google Scholar]
- NOVIKOFF A. B., ESSNER E. Cytolysomes and mitochondrial degeneration. J Cell Biol. 1962 Oct;15:140–146. doi: 10.1083/jcb.15.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALADE G. E. A small particulate component of the cytoplasm. J Biophys Biochem Cytol. 1955 Jan;1(1):59–68. doi: 10.1083/jcb.1.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALADE G. E. Intracisternal granules in the exocrine cells of the pancreas. J Biophys Biochem Cytol. 1956 Jul 25;2(4):417–422. doi: 10.1083/jcb.2.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHTER G. W. A study of hemosiderosis with the aid of electron microscopy; with observations on the relationship between hemosiderin and ferritin. J Exp Med. 1957 Aug 1;106(2):203–218. doi: 10.1084/jem.106.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCARPELLI D. G., HESS R., PEARSE A. G. The cytochemical localization of oxidative enzymes. I. Diphosphopyridine nucleotide diaphorase and triphosphopyridine nucleotide diaphorase. J Biophys Biochem Cytol. 1958 Nov 25;4(6):747–752. doi: 10.1083/jcb.4.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk B. W., Lazarus S. S. Rabbit Pancreas in Protein Malnutrition (Experimental Kwashiorkor) and after Cortisone Administration. Am J Pathol. 1960 Aug;37(2):121–135. [PMC free article] [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Cellular changes accompanying the degenerative and regenerative phase of ethionine-induced pancreatic damage in the rat. Lab Invest. 1953 Jul-Aug;2(4):253–265. [PubMed] [Google Scholar]
- WEISBLUM B., HERMAN L., FITZGERALD P. J. Changes in pancreatic acinar cells during protein deprivation. J Cell Biol. 1962 Feb;12:313–327. doi: 10.1083/jcb.12.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]