Abstract
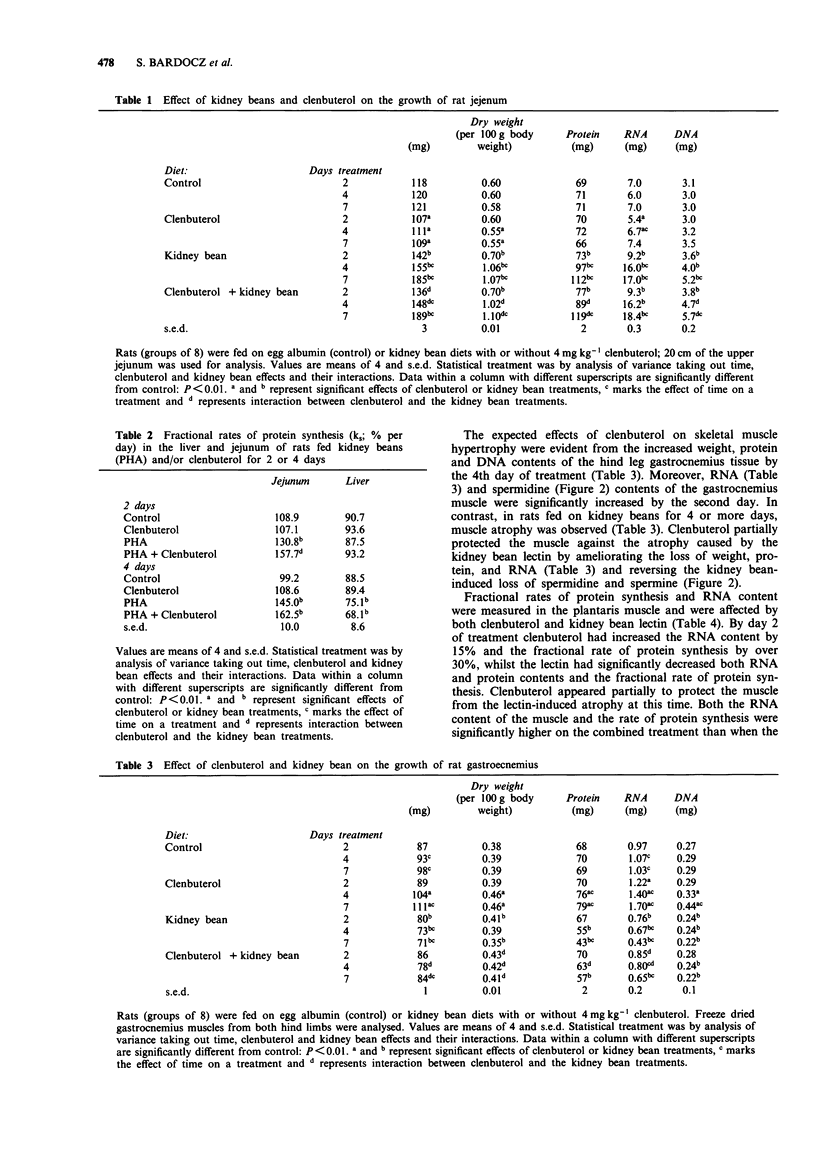
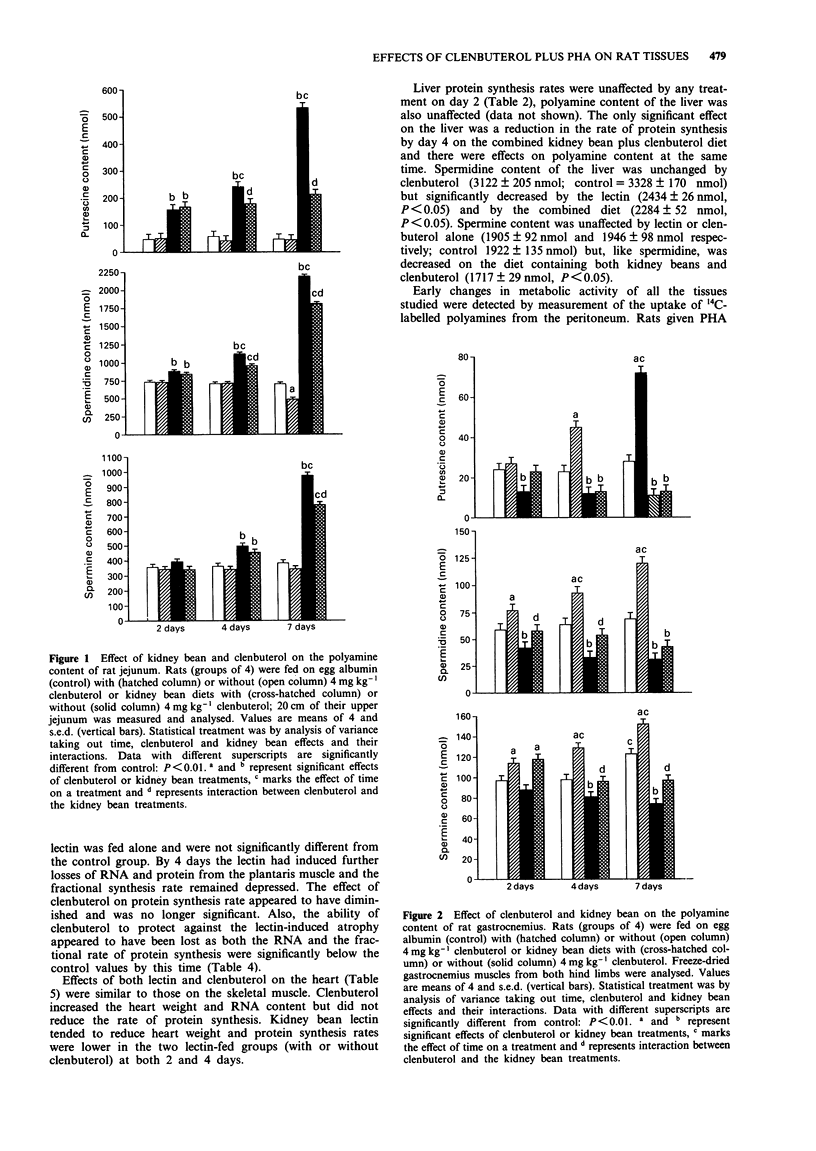
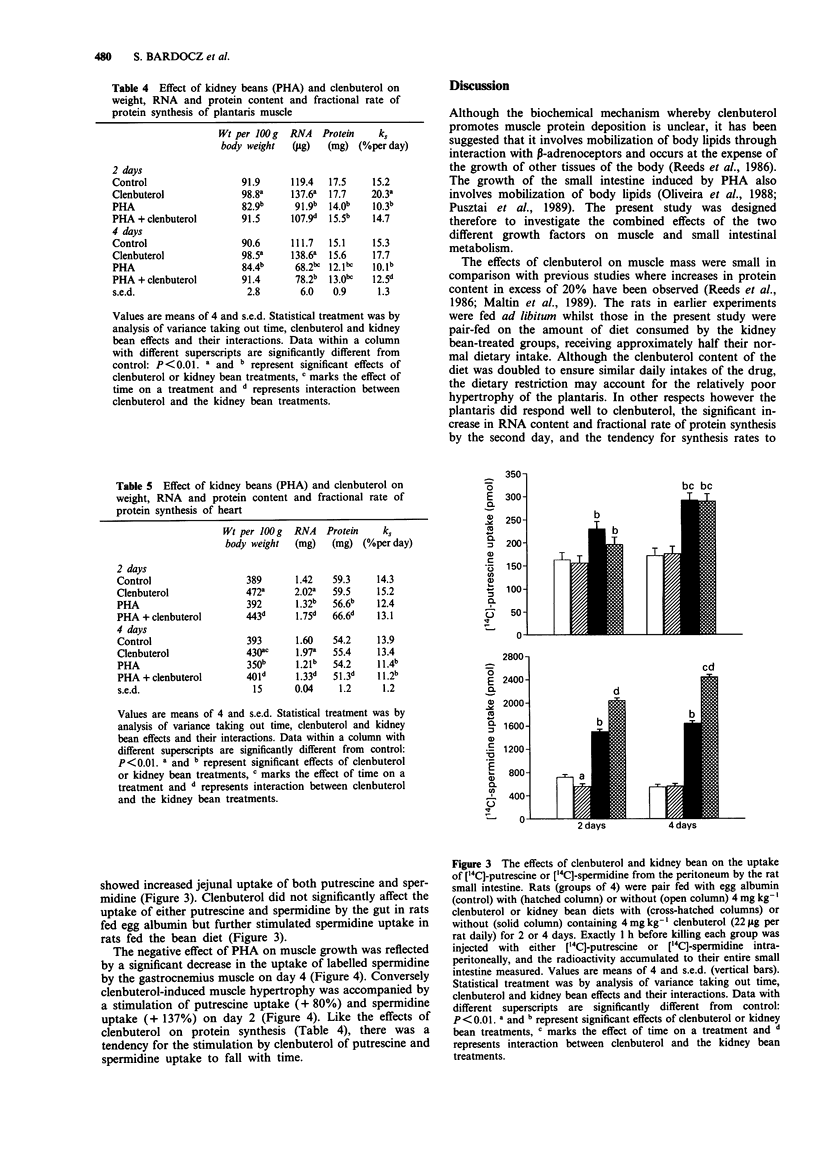
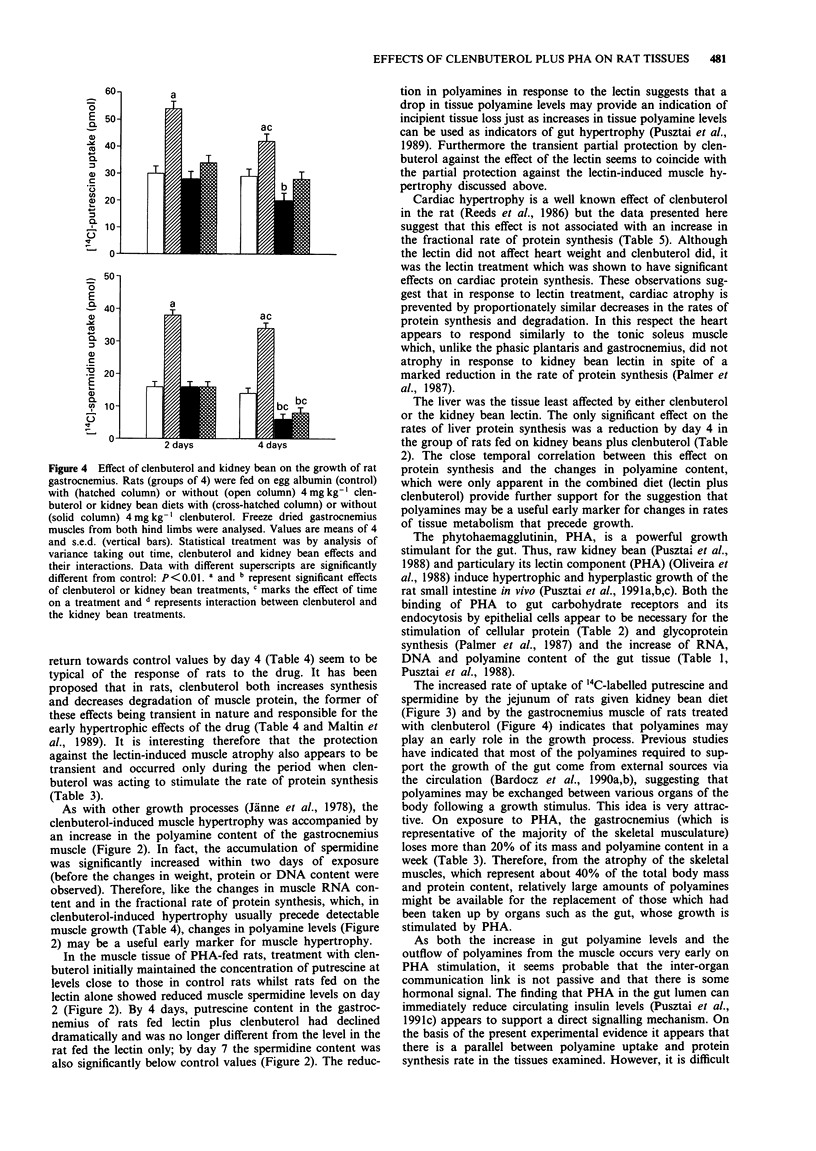
1. The kidney bean lectin, phytohaemagglutinin (PHA), induced a marked atrophy of skeletal muscle which was evident from the changes in tissue composition (protein, RNA, DNA and polyamine content) and from the reduction in weight and protein synthesis of hind leg muscles of rats fed on kidney bean-diets for four days. The beta-adrenoceptor agonist, clenbuterol, induced skeletal muscle hypertrophy by transiently stimulating protein synthesis. As a consequence, the muscle loss caused by a short exposure to PHA was, in part, ameliorated by clenbuterol treatment. 2. Cardiac muscle was affected to a lesser extent than skeletal muscle by both clenbuterol and the lectin. However, there was evidence that protein synthesis in heart was reduced by PHA. 3. PHA had opposite effects on the gut, the lectin-induced hyperplasia of the jejunum was accompanied by a large increase in protein synthesis. Clenbuterol alone had no effect on the jejunum whereas a combination of PHA and clenbuterol appeared to exacerbate the effect of the lectin on gut. 4. Both the lectin-induced gut growth and the hypertrophy of skeletal muscle caused by clenbuterol were preceded by the accumulation of polyamines in the respective tissues. Of particular note was the observation that a significant increase in the proportion of the intraperitoneally injected 14C-labelled spermidine or putrescine taken up by the growing tissues could be detected by the second day. Therefore, the measurement of uptake of labelled polyamines may be used as a sensitive indicator of early alterations in tissue metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardocz S., Brown D. S., Grant G., Pusztai A. Luminal and basolateral polyamine uptake by rat small intestine stimulated to grow by Phaseolus vulgaris lectin phytohaemagglutinin in vivo. Biochim Biophys Acta. 1990 Apr 23;1034(1):46–52. doi: 10.1016/0304-4165(90)90151-l. [DOI] [PubMed] [Google Scholar]
- Bardócz S., Grant G., Brown D. S., Ewen S. W., Nevison I., Pusztai A. Polyamine metabolism and uptake during Phaseolus vulgaris lectin, PHA-induced growth of rat small intestine. Digestion. 1990;46 (Suppl 2):360–366. doi: 10.1159/000200409. [DOI] [PubMed] [Google Scholar]
- Clowes G. H., Jr, George B. C., Villee C. A., Jr, Saravis C. A. Muscle proteolysis induced by a circulating peptide in patients with sepsis or trauma. N Engl J Med. 1983 Mar 10;308(10):545–552. doi: 10.1056/NEJM198303103081001. [DOI] [PubMed] [Google Scholar]
- Emery P. W., Rothwell N. J., Stock M. J., Winter P. D. Chronic effects of beta 2-adrenergic agonists on body composition and protein synthesis in the rat. Biosci Rep. 1984 Jan;4(1):83–91. doi: 10.1007/BF01120827. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson M. M., Pell J. M., Bates P. C., Millward D. J. The effects of endotoxaemia on protein metabolism in skeletal muscle and liver of fed and fasted rats. Biochem J. 1986 Apr 15;235(2):329–336. doi: 10.1042/bj2350329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- King T. P., Pusztai A., Grant G., Slater D. Immunogold localization of ingested kidney bean (Phaseolus vulgaris) lectins in epithelial cells of the rat small intestine. Histochem J. 1986 Aug;18(8):413–420. doi: 10.1007/BF01675333. [DOI] [PubMed] [Google Scholar]
- LOVTRUP S., ROOS K. Observations on the chemical determination of deoxyribonucleic acid in animal tissues. Biochim Biophys Acta. 1961 Oct 14;53:1–10. doi: 10.1016/0006-3002(61)90788-0. [DOI] [PubMed] [Google Scholar]
- Maltin C. A., Hay S. M., Delday M. I., Lobley G. E., Reeds P. J. The action of the beta-agonist clenbuterol on protein metabolism in innervated and denervated phasic muscles. Biochem J. 1989 Aug 1;261(3):965–971. doi: 10.1042/bj2610965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltin C. A., Hay S. M., Delday M. I., Smith F. G., Lobley G. E., Reeds P. J. Clenbuterol, a beta agonist, induces growth in innervated and denervated rat soleus muscle via apparently different mechanisms. Biosci Rep. 1987 Jun;7(6):525–532. doi: 10.1007/BF01116510. [DOI] [PubMed] [Google Scholar]
- McElligott M. A., Mulder J. E., Chaung L. Y., Barreto A., Jr Clenbuterol-induced muscle growth: investigation of possible mediation by insulin. Am J Physiol. 1987 Oct;253(4 Pt 1):E370–E375. doi: 10.1152/ajpendo.1987.253.4.E370. [DOI] [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., Nnanyelugo D. O., Waterlow J. C. The relative importance of muscle protein synthesis and breakdown in the regulation of muscle mass. Biochem J. 1976 Apr 15;156(1):185–188. doi: 10.1042/bj1560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Delday M. I., McMillan D. N., Noble B. S., Bain P., Maltin C. A. Effects of the cyclo-oxygenase inhibitor, fenbufen, on clenbuterol-induced hypertrophy of cardiac and skeletal muscle of rats. Br J Pharmacol. 1990 Dec;101(4):835–838. doi: 10.1111/j.1476-5381.1990.tb14166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztai A., Ewen S. W., Grant G., Peumans W. J., van Damme E. J., Rubio L., Bardocz S. Relationship between survival and binding of plant lectins during small intestinal passage and their effectiveness as growth factors. Digestion. 1990;46 (Suppl 2):308–316. doi: 10.1159/000200402. [DOI] [PubMed] [Google Scholar]
- Reeds P. J., Hay S. M., Dorwood P. M., Palmer R. M. Stimulation of muscle growth by clenbuterol: lack of effect on muscle protein biosynthesis. Br J Nutr. 1986 Jul;56(1):249–258. doi: 10.1079/bjn19860104. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Seiler N., Knödgen B. High-performance liquid chromatographic procedure for the simultaneous determination of the natural polyamines and their monoacetyl derivatives. J Chromatogr. 1980 Dec 12;221(2):227–235. doi: 10.1016/s0378-4347(00)84307-8. [DOI] [PubMed] [Google Scholar]
- Williams P. E., Pagliani L., Innes G. M., Pennie K., Harris C. I., Garthwaite P. Effects of a beta-agonist (clenbuterol) on growth, carcass composition, protein and energy metabolism of veal calves. Br J Nutr. 1987 May;57(3):417–428. doi: 10.1079/bjn19870049. [DOI] [PubMed] [Google Scholar]


