Abstract
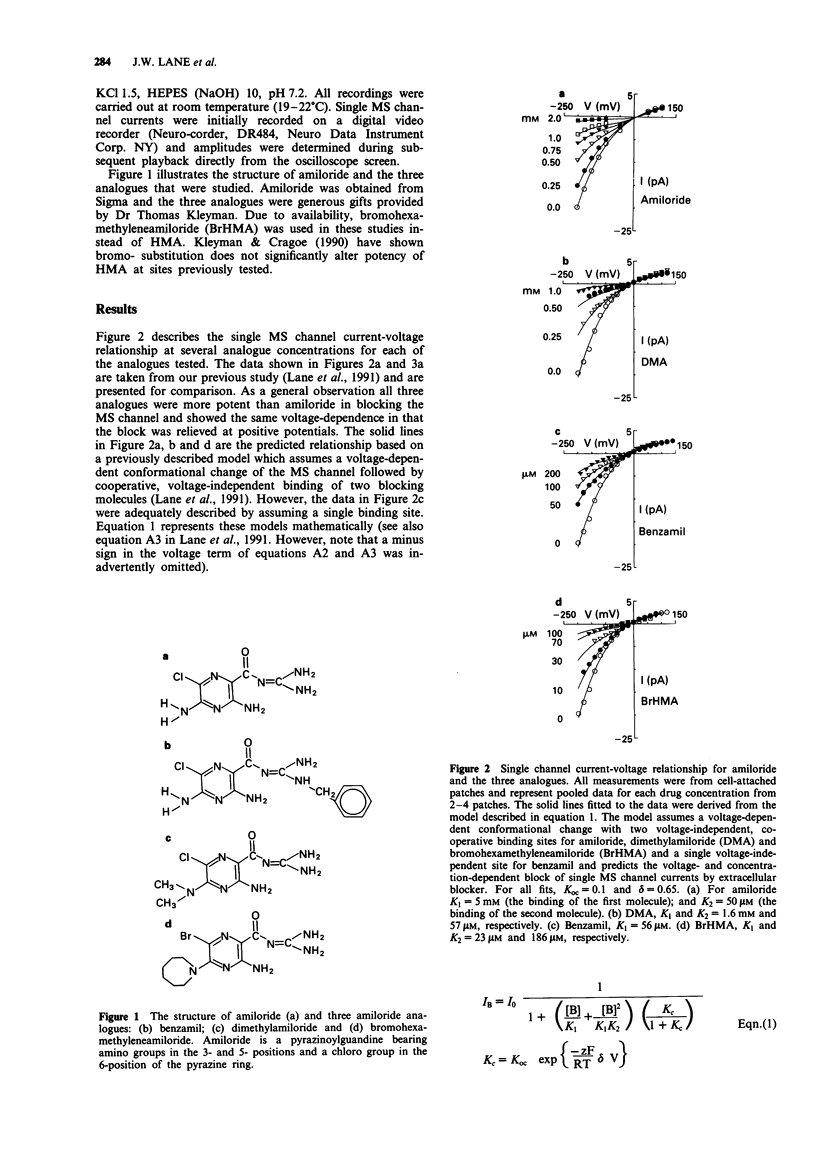
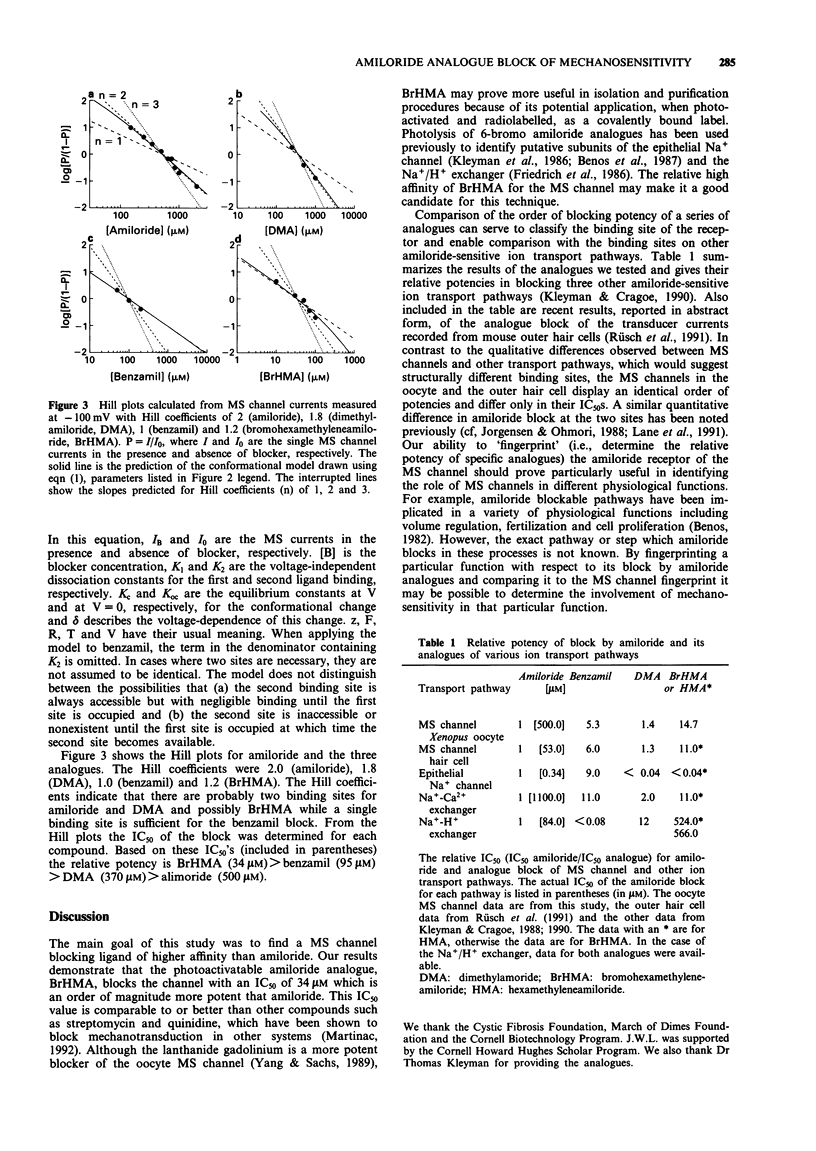
1. Patch clamp recording techniques have been used to compare the block caused by amiloride and some of its structural analogues of the mechanosensitive (MS) cation selective channel in frog (Xenopus laevis) oocytes. 2. Like amiloride, the amiloride analogues dimethylamiloride (DMA), benzamil and bromohexamethyleneamiloride (BrHMA) block the MS channel in a highly voltage-dependent manner. 3. All analogues tested were more potent blockers than amiloride with IC50's of 500 microM (amiloride), 370 microM (DMA), 95 microM (benzamil) and 34 microM (BrHMA). 4. Hill plots gave Hill coefficients of 2 (amiloride), 1.8 (DMA), 1 (benzamil) and 1.2 (BrHMA) indicating that the binding of two ligand molecules may be necessary for the block caused by amiloride, DMA and possibly BrHMA whereas only a single ligand molecule may be required for the block by benzamil. 5. The potential use of BrHMA as a light-activated, covalent label of the MS channel protein is discussed. 6. The amiloride analogue 'fingerprinting' of the blocking site on the MS channel indicates it is structurally different from previously described amiloride-sensitive ion transport pathways but may be related to the amiloride binding site on outer hair cells of the ear.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew W. S., Levinson S. R., Brabson J. S., Raftery M. A. Purification of the tetrodotoxin-binding component associated with the voltage-sensitive sodium channel from Electrophorus electricus electroplax membranes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2606–2610. doi: 10.1073/pnas.75.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benos D. J. Amiloride: a molecular probe of sodium transport in tissues and cells. Am J Physiol. 1982 Mar;242(3):C131–C145. doi: 10.1152/ajpcell.1982.242.3.C131. [DOI] [PubMed] [Google Scholar]
- Benos D. J., Saccomani G., Sariban-Sohraby S. The epithelial sodium channel. Subunit number and location of the amiloride binding site. J Biol Chem. 1987 Aug 5;262(22):10613–10618. [PubMed] [Google Scholar]
- Changeux J. P., Kasai M., Lee C. Y. Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1241–1247. doi: 10.1073/pnas.67.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen O. Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature. 1987 Nov 5;330(6143):66–68. doi: 10.1038/330066a0. [DOI] [PubMed] [Google Scholar]
- Friedrich T., Sablotni J., Burckhardt G. Identification of the renal Na+/H+ exchanger with N,N'-dicyclohexylcarbodiimide (DCCD) and amiloride analogues. J Membr Biol. 1986;94(3):253–266. doi: 10.1007/BF01869721. [DOI] [PubMed] [Google Scholar]
- Graham D., Pfeiffer F., Betz H. UV light-induced cross-linking of strychnine to the glycine receptor of rat spinal cord membranes. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1330–1335. doi: 10.1016/s0006-291x(81)80157-x. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. The Sharpey-Schafer Lecture. Ionic channels: evolutionary origins and modern roles. Q J Exp Physiol. 1989 Nov;74(6):785–804. doi: 10.1113/expphysiol.1989.sp003349. [DOI] [PubMed] [Google Scholar]
- Jørgensen F., Ohmori H. Amiloride blocks the mechano-electrical transduction channel of hair cells of the chick. J Physiol. 1988 Sep;403:577–588. doi: 10.1113/jphysiol.1988.sp017265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyman T. R., Cragoe E. J., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988 Oct;105(1):1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Kleyman T. R., Cragoe E. J., Jr Cation transport probes: the amiloride series. Methods Enzymol. 1990;191:739–755. doi: 10.1016/0076-6879(90)91045-8. [DOI] [PubMed] [Google Scholar]
- Kleyman T. R., Yulo T., Ashbaugh C., Landry D., Cragoe E., Jr, Karlin A., Al-Awqati Q. Photoaffinity labeling of the epithelial sodium channel. J Biol Chem. 1986 Feb 25;261(6):2839–2843. [PubMed] [Google Scholar]
- Lane J. W., McBride D. W., Jr, Hamill O. P. Amiloride block of the mechanosensitive cation channel in Xenopus oocytes. J Physiol. 1991 Sep;441:347–366. doi: 10.1113/jphysiol.1991.sp018755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B., Buechner M., Delcour A. H., Adler J., Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986 Dec;407(6):577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989 Feb 24;243(4894 Pt 1):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]


