Abstract
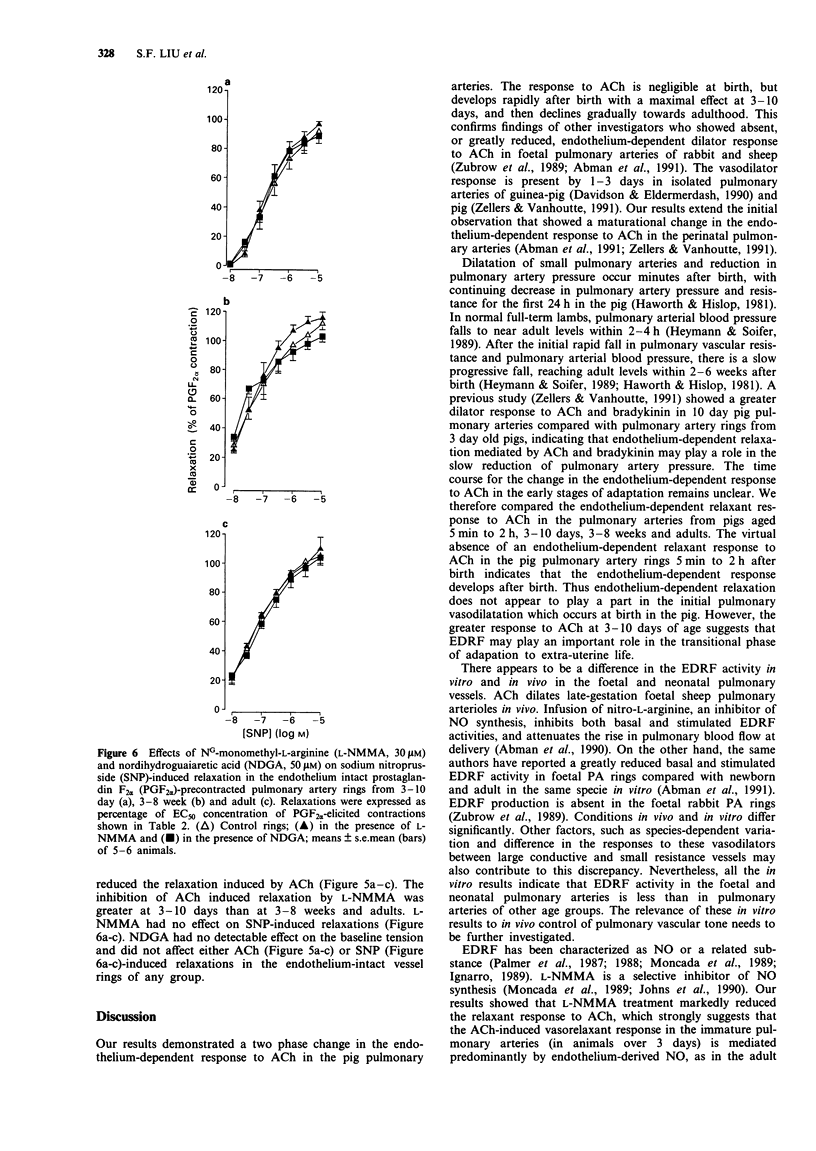
1. We compared in vitro endothelium-dependent vasorelaxant responses to acetylcholine (ACh) and the endothelium-independent vasodilator response to sodium nitroprusside (SNP) in prostaglandin F2 alpha (PGF2 alpha)-precontracted muscular pulmonary arteries (PA) from pigs aged 5 min to 2 h (neonatal), 3-10 days, 3-8 weeks and adults. 2. In the pulmonary artery (PA) rings from neonatal animals, the vasodilator response to ACh was negligible. However, responses to ACh were present in all PA rings from older animals, being greatest at 3-10 days and then decreasing with age (P less than 0.001, ANOVA). ACh (30 microM) induced a 1 +/- 1%, 92 +/- 9%, 62 +/- 5% and 51 +/- 6% reduction of the PGF2 alpha-generated tension in neonatal, 3-10 days, 3-8 weeks and adult groups, respectively. 3. The relaxant response to SNP was present in the PA rings from all age groups and increased with age (P less than 0.001, ANOVA). SNP (1 microM)-induced relaxation was 55 +/- 9%, 73 +/- 7%, 97 +/- 5% and 93 +/- 6% in neonatal, 3-10 days, 3-8 week and adult groups, respectively. 4. Removal of the vascular endothelium abolished the relaxant response to ACh but had no effect on the response to SNP in any groups. 5. NG-monomethyl-L-arginine (30 microM), a nitric oxide synthesis inhibitor, inhibited the response to ACh but not to SNP. The lipoxygenase inhibitor, nordihydroguaiaretic acid, had no significant effect on responses to ACh or SNP in any group.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abman S. H., Chatfield B. A., Hall S. L., McMurtry I. F. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol. 1990 Dec;259(6 Pt 2):H1921–H1927. doi: 10.1152/ajpheart.1990.259.6.H1921. [DOI] [PubMed] [Google Scholar]
- Abman S. H., Chatfield B. A., Rodman D. M., Hall S. L., McMurtry I. F. Maturational changes in endothelium-derived relaxing factor activity of ovine pulmonary arteries in vitro. Am J Physiol. 1991 Apr;260(4 Pt 1):L280–L285. doi: 10.1152/ajplung.1991.260.4.L280. [DOI] [PubMed] [Google Scholar]
- Allen K., Haworth S. G. Human postnatal pulmonary arterial remodeling. Ultrastructural studies of smooth muscle cell and connective tissue maturation. Lab Invest. 1988 Nov;59(5):702–709. [PubMed] [Google Scholar]
- Amezcua J. L., Dusting G. J., Palmer R. M., Moncada S. Acetylcholine induces vasodilatation in the rabbit isolated heart through the release of nitric oxide, the endogenous nitrovasodilator. Br J Pharmacol. 1988 Nov;95(3):830–834. doi: 10.1111/j.1476-5381.1988.tb11711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaraman V., Kullama L. K., Easa D., Robillard J. E., Hashiro G. M., Nakamura K. T. Developmental changes in sodium nitroprusside and atrial natriuretic factor mediated relaxation in the guinea pig aorta. Pediatr Res. 1990 Apr;27(4 Pt 1):392–395. doi: 10.1203/00006450-199004000-00013. [DOI] [PubMed] [Google Scholar]
- Crawley D. E., Liu S. F., Evans T. W., Barnes P. J. Inhibitory role of endothelium-derived relaxing factor in rat and human pulmonary arteries. Br J Pharmacol. 1990 Sep;101(1):166–170. doi: 10.1111/j.1476-5381.1990.tb12107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson D. Circulating vasoactive substances and hemodynamic adjustments at birth in lambs. J Appl Physiol (1985) 1987 Aug;63(2):676–684. doi: 10.1152/jappl.1987.63.2.676. [DOI] [PubMed] [Google Scholar]
- Davidson D., Eldemerdash A. Endothelium-derived relaxing factor: presence in pulmonary and systemic arteries of the newborn guinea pig. Pediatr Res. 1990 Feb;27(2):128–132. doi: 10.1203/00006450-199002000-00005. [DOI] [PubMed] [Google Scholar]
- Davidson D. Pulmonary hemodynamics at birth: effect of acute cyclooxygenase inhibition in lambs. J Appl Physiol (1985) 1988 Apr;64(4):1676–1682. doi: 10.1152/jappl.1988.64.4.1676. [DOI] [PubMed] [Google Scholar]
- Dunn J. A., Lorch V., Sinha S. N. Responses of small intrapulmonary arteries to vasoactive compounds in the fetal and neonatal lamb: norepinephrine, epinephrine, serotonin, and potassium chloride. Pediatr Res. 1989 Apr;25(4):360–363. doi: 10.1203/00006450-198904000-00009. [DOI] [PubMed] [Google Scholar]
- Feddersen C. O., Mathias M. M., McMurtry I. F., Voelkel N. F. Acetylcholine induces vasodilation and prostacyclin synthesis in rat lungs. Prostaglandins. 1986 May;31(5):973–987. doi: 10.1016/0090-6980(86)90027-4. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ Res. 1986 Apr;58(4):531–538. doi: 10.1161/01.res.58.4.531. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Neufang B. The endothelium-dependent vasodilator effect of acetylcholine: characterization of the endothelial relaxing factor with inhibitors of arachidonic acid metabolism. Eur J Pharmacol. 1984 Aug 3;103(1-2):65–70. doi: 10.1016/0014-2999(84)90190-0. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990 May;15(5):486–492. doi: 10.1161/01.hyp.15.5.486. [DOI] [PubMed] [Google Scholar]
- Greenberg B., Rhoden K., Barnes P. J. Endothelium-dependent relaxation of human pulmonary arteries. Am J Physiol. 1987 Feb;252(2 Pt 2):H434–H438. doi: 10.1152/ajpheart.1987.252.2.H434. [DOI] [PubMed] [Google Scholar]
- Hall S. M., Haworth S. G. Conducting pulmonary arteries: structural adaptation to extrauterine life in the pig. Cardiovasc Res. 1987 Mar;21(3):208–216. doi: 10.1093/cvr/21.3.208. [DOI] [PubMed] [Google Scholar]
- Hall S. M., Haworth S. G. Normal adaptation of pulmonary arterial intima to extrauterine life in the pig: ultrastructural studies. J Pathol. 1986 May;149(1):55–66. doi: 10.1002/path.1711490111. [DOI] [PubMed] [Google Scholar]
- Haworth S. G., Hislop A. A. Adaptation of the pulmonary circulation to extra-uterine life in the pig and its relevance to the human infant. Cardiovasc Res. 1981 Feb;15(2):108–119. doi: 10.1093/cvr/15.2.108. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989 Jul;65(1):1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- Johns R. A., Peach M. J., Linden J., Tichotsky A. NG-monomethyl L-arginine inhibits endothelium-derived relaxing factor-stimulated cyclic GMP accumulation in cocultures of endothelial and vascular smooth muscle cells by an action specific to the endothelial cell. Circ Res. 1990 Oct;67(4):979–985. doi: 10.1161/01.res.67.4.979. [DOI] [PubMed] [Google Scholar]
- Leffler C. W., Hessler J. R. Perinatal pulmonary prostaglandin production. Am J Physiol. 1981 Nov;241(5):H756–H759. doi: 10.1152/ajpheart.1981.241.5.H756. [DOI] [PubMed] [Google Scholar]
- Leffler C. W., Tyler T. L., Cassin S. Effect of indomethacin on pulmonary vascular response to ventilation of fetal goats. Am J Physiol. 1978 Apr;234(4):H346–H351. doi: 10.1152/ajpheart.1978.234.4.H346. [DOI] [PubMed] [Google Scholar]
- Liu S. F., Crawley D. E., Barnes P. J., Evans T. W. Endothelium-derived relaxing factor inhibits hypoxic pulmonary vasoconstriction in rats. Am Rev Respir Dis. 1991 Jan;143(1):32–37. doi: 10.1164/ajrccm/143.1.32. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Newman J. H., Souhrada J. F., Reeves J. T., Arroyave C. M., Grover R. F. Postnatal changes in response of canine neonatal pulmonary arteries to histamine. Am J Physiol. 1979 Jul;237(1):H76–H82. doi: 10.1152/ajpheart.1979.237.1.H76. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer H. A., Peach M. J. Endothelium-dependent relaxation of rabbit aorta. II. Inhibition of relaxation stimulated by methacholine and A23187 with antagonists of arachidonic acid metabolism. J Pharmacol Exp Ther. 1983 Sep;226(3):796–801. [PubMed] [Google Scholar]
- Su C., Bevan J. A., Assali N. S., Brinkman C. R., 3rd Regional variation of lamb blood vessel responsiveness to vasoactive agents during fetal development. Circ Res. 1977 Dec;41(6):844–848. doi: 10.1161/01.res.41.6.844. [DOI] [PubMed] [Google Scholar]
- Teitel D. F., Iwamoto H. S., Rudolph A. M. Changes in the pulmonary circulation during birth-related events. Pediatr Res. 1990 Apr;27(4 Pt 1):372–378. doi: 10.1203/00006450-199004000-00010. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Wharton J., Haworth S. G., Polak J. M. Postnatal development of the innervation and paraganglia in the porcine pulmonary arterial bed. J Pathol. 1988 Jan;154(1):19–27. doi: 10.1002/path.1711540104. [DOI] [PubMed] [Google Scholar]
- Zellers T. M., Vanhoutte P. M. Endothelium-dependent relaxations of piglet pulmonary arteries augment with maturation. Pediatr Res. 1991 Aug;30(2):176–180. doi: 10.1203/00006450-199108000-00011. [DOI] [PubMed] [Google Scholar]



