Abstract
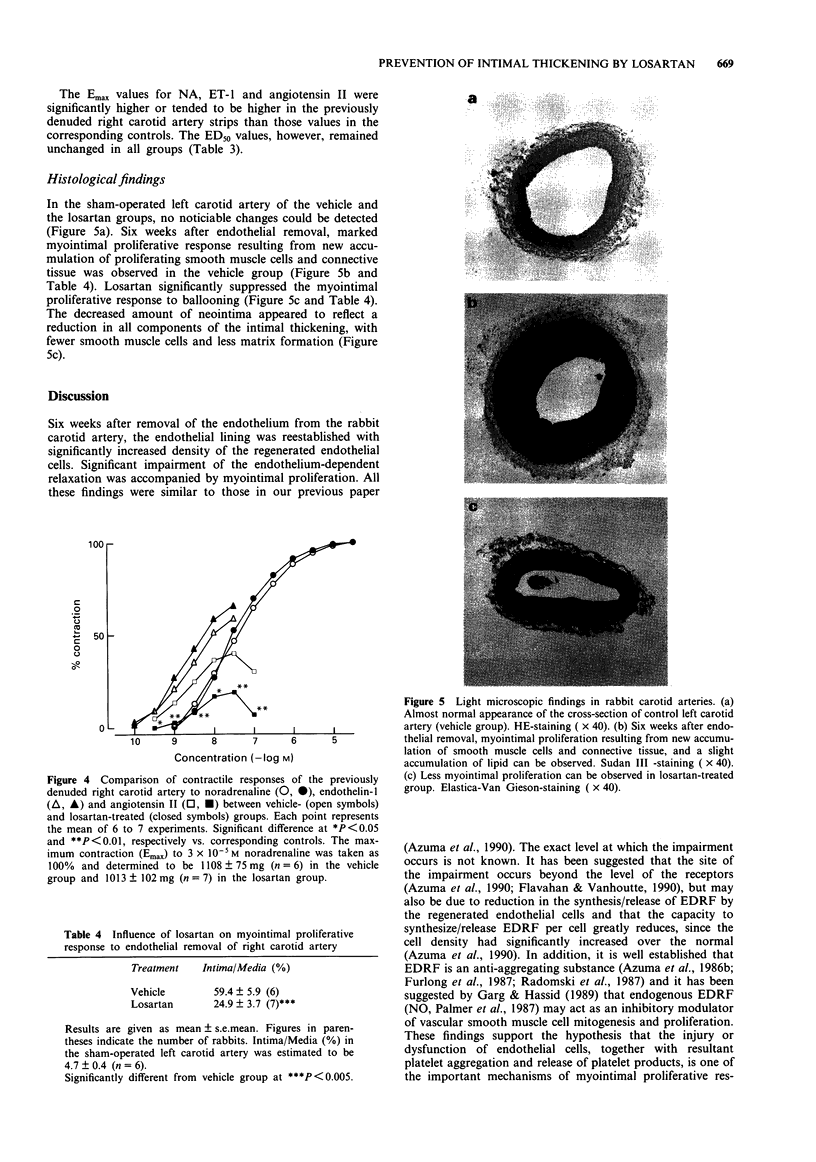
1. The present experiments were designed to investigate the role of local angiotensin II receptors in the myointimal proliferative response of the vascular wall after endothelial removal, by use of a novel, nonpeptide, angiotensin II receptor antagonist, losartan. 2. When administered 1 week before endothelial removal from the rabbit carotid artery and then continuously until animals were killed 6 weeks later, losartan in a dose of 10 mg kg-1 daily, p.o. had no significant effects on the carotid blood flow (CBF), mean arterial blood pressure (MBP) and heart rate (HR). 3. A full endothelial lining with increased density of regenerated endothelial cells was observed 6 weeks after the endothelial removal. These changes were unaffected by treatment with losartan. 4. Six weeks after endothelial removal, acetylcholine (ACh)- and adenosine diphosphate (ADP)-induced relaxations were greatly reduced though endothelial cells had regenerated. The reduction of the relaxations to these agonists were significantly restored by chronic treatment with losartan. The endothelial-independent, sodium nitroprusside (SNP)-induced relaxation remained unaffected in all groups. 5. There were no differences in the noradrenaline (NA)- and endothelin-1 (ET-1)-induced contractions of the carotid artery strips between vehicle and losartan-treated groups. In contrast, the contractile response of the strips to angiotensin II was significantly decreased in the losartan group, indicating the specific antagonism by chronic losartan against the angiotensin II receptor. 6. Six weeks after endothelial removal, marked myointimal proliferation resulting from new accumulation of proliferating smooth muscle cells and connective tissue was observed in the vehicle group. Losartan treatment greatly suppressed the myointimal proliferative response.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
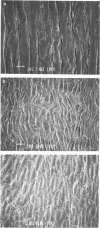
PDF



Images in this article
Selected References
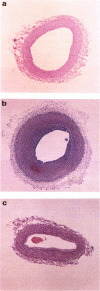
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma H., Funayama N., Kubota T., Ishikawa M. Regeneration of endothelial cells after balloon denudation of the rabbit carotid artery and changes in responsiveness. Jpn J Pharmacol. 1990 Apr;52(4):541–552. doi: 10.1254/jjp.52.541. [DOI] [PubMed] [Google Scholar]
- Azuma H., Ishikawa M., Nakajima T., Satoh A., Sekizaki S. Calcium-dependent contractile response of arterial smooth muscle to a jellyfish toxin (pCrTX: Carybdea rastonii). Br J Pharmacol. 1986 Jul;88(3):549–559. doi: 10.1111/j.1476-5381.1986.tb10235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H., Ishikawa M., Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986 Jun;88(2):411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H., Sugimoto-Tokushima M., Tanaka K., Ikenoue Y., Ito S., Ishikawa M. Alpha 1-adrenoceptor antagonist activity of novel pyrimidine derivatives (SHI437 and IK29) in rabbit aorta and trigone of the bladder. Br J Pharmacol. 1989 Apr;96(4):1000–1006. doi: 10.1111/j.1476-5381.1989.tb11913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassis L. A., Saye J., Peach M. J. Location and regulation of rat angiotensinogen messenger RNA. Hypertension. 1988 Jun;11(6 Pt 2):591–596. doi: 10.1161/01.hyp.11.6.591. [DOI] [PubMed] [Google Scholar]
- Chiu A. T., McCall D. E., Price W. A., Wong P. C., Carini D. J., Duncia J. V., Wexler R. R., Yoo S. E., Johnson A. L., Timmermans P. B. Nonpeptide angiotensin II receptor antagonists. VII. Cellular and biochemical pharmacology of DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther. 1990 Feb;252(2):711–718. [PubMed] [Google Scholar]
- Daemen M. J., Lombardi D. M., Bosman F. T., Schwartz S. M. Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res. 1991 Feb;68(2):450–456. doi: 10.1161/01.res.68.2.450. [DOI] [PubMed] [Google Scholar]
- Flavahan N. A., Vanhoutte P. M. G-proteins and endothelial responses. Blood Vessels. 1990;27(2-5):218–229. doi: 10.1159/000158813. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Furlong B., Henderson A. H., Lewis M. J., Smith J. A. Endothelium-derived relaxing factor inhibits in vitro platelet aggregation. Br J Pharmacol. 1987 Apr;90(4):687–692. doi: 10.1111/j.1476-5381.1987.tb11221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisterfer A. A., Peach M. J., Owens G. K. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988 Apr;62(4):749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Takagi Y., Fukuda Y., Marumo F. Endothelin is a potent mitogen for rat vascular smooth muscle cells. Atherosclerosis. 1989 Aug;78(2-3):225–228. doi: 10.1016/0021-9150(89)90227-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Hattori K. Nitroarginine inhibits endothelium-derived relaxation. Jpn J Pharmacol. 1990 Jan;52(1):167–169. doi: 10.1254/jjp.52.167. [DOI] [PubMed] [Google Scholar]
- Luft F. C., Wilcox C. S., Unger T., Kühn R., Demmert G., Rohmeiss P., Ganten D., Sterzel R. B. Angiotensin-induced hypertension in the rat. Sympathetic nerve activity and prostaglandins. Hypertension. 1989 Oct;14(4):396–403. doi: 10.1161/01.hyp.14.4.396. [DOI] [PubMed] [Google Scholar]
- Lyall F., Morton J. J., Lever A. F., Cragoe E. J. Angiotensin II activates Na+-H+ exchange and stimulates growth in cultured vascular smooth muscle cells. J Hypertens Suppl. 1988 Dec;6(4):S438–S441. doi: 10.1097/00004872-198812040-00138. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Daemen M. J., Schwartz S. M. Alpha 1-adrenergic stimulation of platelet-derived growth factor A-chain gene expression in rat aorta. J Biol Chem. 1990 Jan 15;265(2):1082–1088. [PubMed] [Google Scholar]
- Nakamura Y., Nakamura K., Matsukura T., Nakamura K. Vascular angiotensin converting enzyme activity in spontaneously hypertensive rats and its inhibition with cilazapril. J Hypertens. 1988 Feb;6(2):105–110. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Penit J., Faure M., Jard S. Vasopressin and angiotensin II receptors in rat aortic smooth muscle cells in culture. Am J Physiol. 1983 Jan;244(1):E72–E82. doi: 10.1152/ajpendo.1983.244.1.E72. [DOI] [PubMed] [Google Scholar]
- Powell J. S., Clozel J. P., Müller R. K., Kuhn H., Hefti F., Hosang M., Baumgartner H. R. Inhibitors of angiotensin-converting enzyme prevent myointimal proliferation after vascular injury. Science. 1989 Jul 14;245(4914):186–188. doi: 10.1126/science.2526370. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resink T. J., Hahn A. W., Scott-Burden T., Powell J., Weber E., Bühler F. R. Inducible endothelin mRNA expression and peptide secretion in cultured human vascular smooth muscle cells. Biochem Biophys Res Commun. 1990 May 16;168(3):1303–1310. doi: 10.1016/0006-291x(90)91171-n. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Gajdusek C. M., Selden S. C., 3rd Vascular wall growth control: the role of the endothelium. Arteriosclerosis. 1981 Mar-Apr;1(2):107–126. doi: 10.1161/01.atv.1.2.107. [DOI] [PubMed] [Google Scholar]
- Taubman M. B., Berk B. C., Izumo S., Tsuda T., Alexander R. W., Nadal-Ginard B. Angiotensin II induces c-fos mRNA in aortic smooth muscle. Role of Ca2+ mobilization and protein kinase C activation. J Biol Chem. 1989 Jan 5;264(1):526–530. [PubMed] [Google Scholar]
- Wong P. C., Price W. A., Chiu A. T., Duncia J. V., Carini D. J., Wexler R. R., Johnson A. L., Timmermans P. B. Nonpeptide angiotensin II receptor antagonists. VIII. Characterization of functional antagonism displayed by DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther. 1990 Feb;252(2):719–725. [PubMed] [Google Scholar]