Abstract
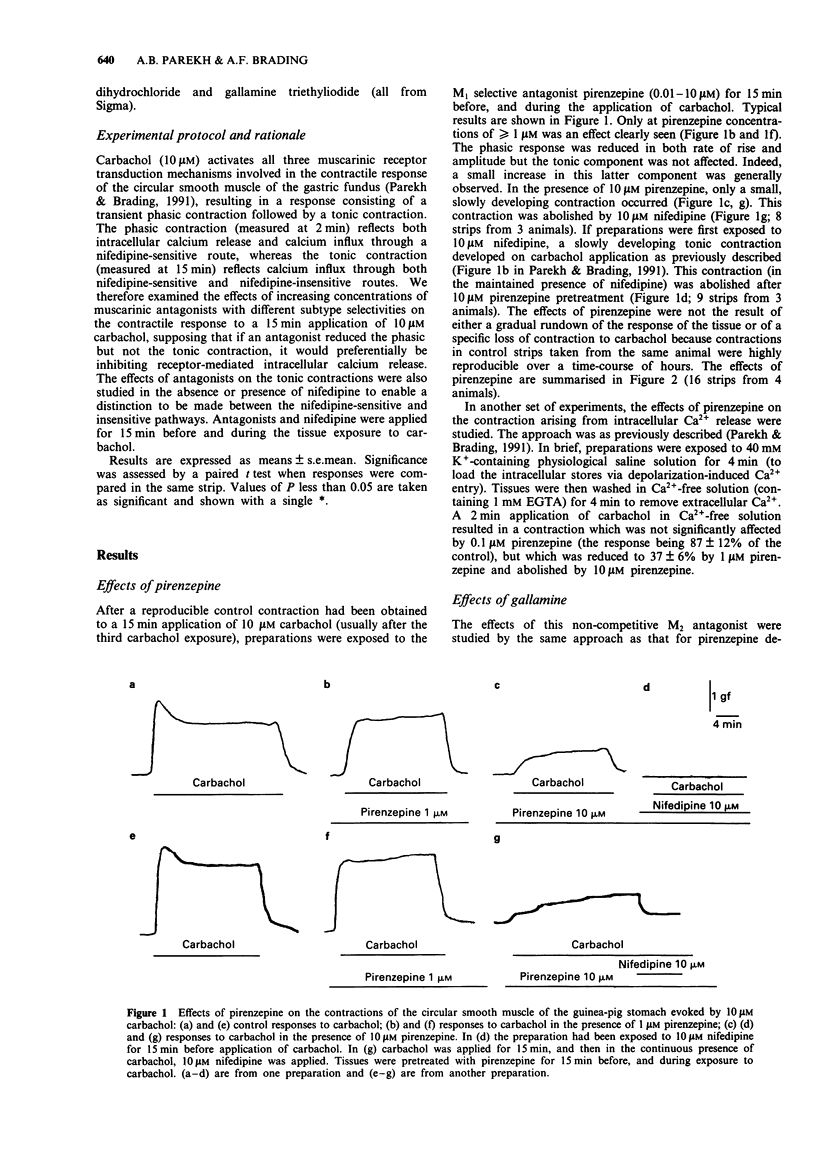
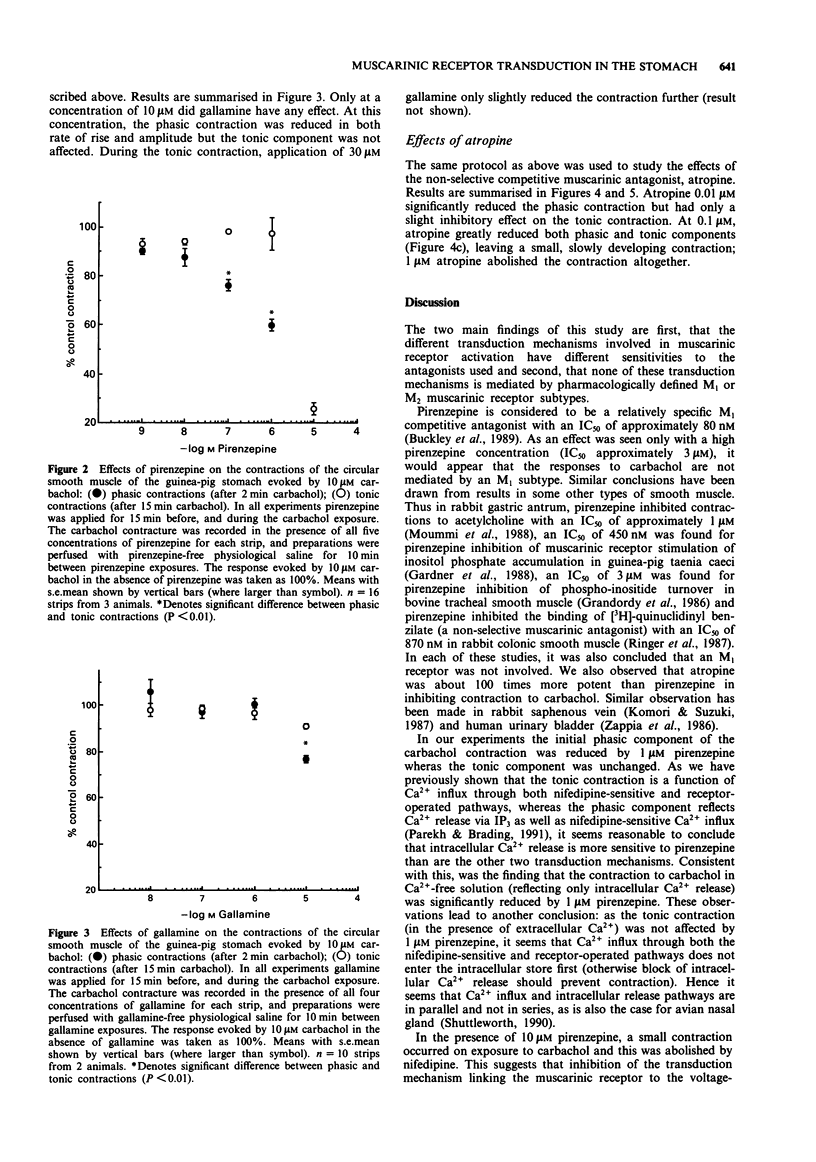
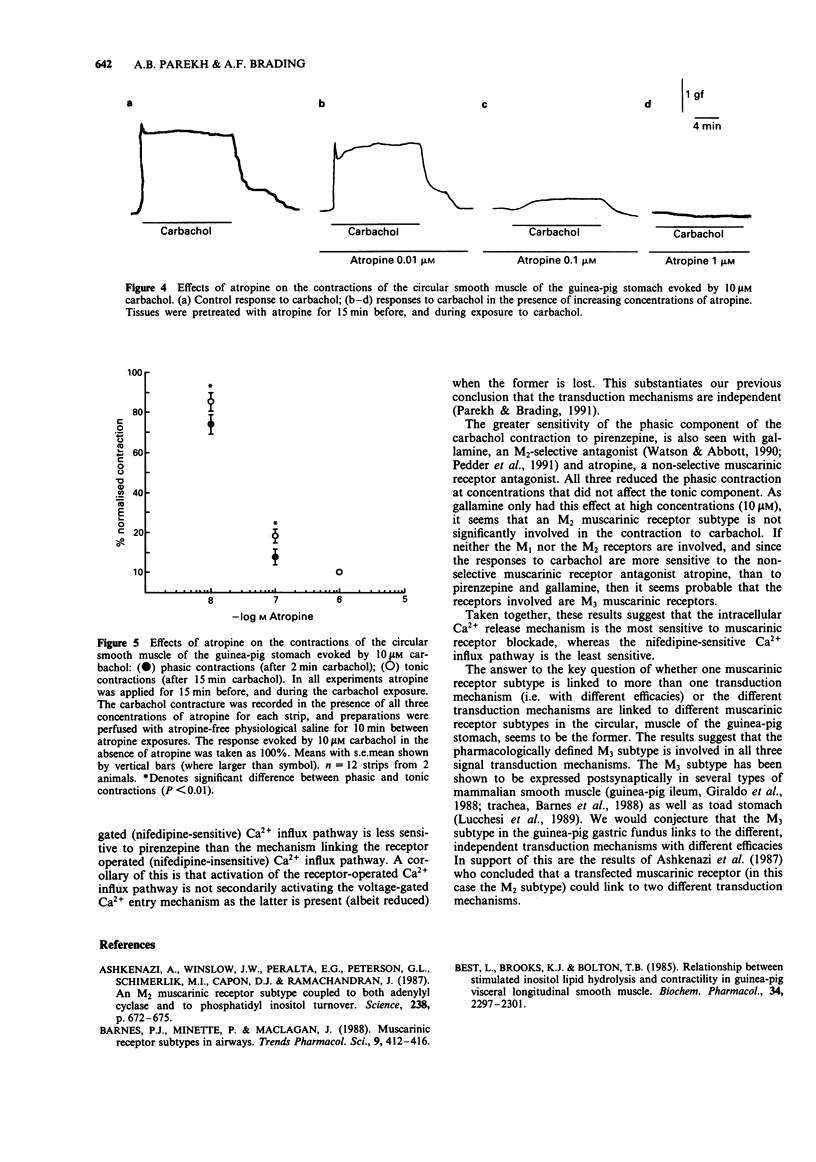
1. In a previous publication, we showed that 10 microM carbachol induced contraction by activating three independent transduction mechanisms in circular smooth muscle of guinea-pig gastric fundus (Parekh & Brading, 1991). These were: inositol trisphosphate-mediated intracellular Ca2+ release, Ca2+ influx through a nifedipine-sensitive route and Ca2+ influx through a receptor operated nifedipine-insensitive pathway. The former two processes contribute to the phasic contraction and the latter two to the tonic contraction. In this paper, we have studied the effects of muscarinic receptor antagonists with known selectivity for different muscarinic receptor subtypes, on the contraction evoked by 10 microM carbachol. 2. Low concentrations of pirenzepine (M1 selective) had little effect on the contraction initiated by carbachol. Higher concentrations (greater than 1 microM) reduced only the phasic component. This concentration of pirenzepine greatly reduced the contraction evoked by 10 microM carbachol in Ca(2+)-free solution, indicating inhibition of intracellular Ca2+ release. 3. In the presence of 10 microM nifedipine, the tonic contraction evoked by 10 microM carbachol (reflecting the receptor-operated nifedipine-insensitive route) was abolished by 10 microM pirenzepine. In the absence of nifedipine pretreatment, however, 10 microM pirenzepine did not abolish the contraction to 10 microM carbachol. This contraction was subsequently abolished by nifedipine. 4. Only high concentrations (greater than 10 microM) of the M2-selective antagonist, gallamine, inhibited the contraction to 10 microM carbachol. Like pirenzepine, gallamine preferentially inhibited the phasic component of the contraction, indicating an effect on intracellular Ca2+ release. 5. The non-selective muscarinic receptor antagonist, atropine, abolished all components of the contraction.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi A., Winslow J. W., Peralta E. G., Peterson G. L., Schimerlik M. I., Capon D. J., Ramachandran J. An M2 muscarinic receptor subtype coupled to both adenylyl cyclase and phosphoinositide turnover. Science. 1987 Oct 30;238(4827):672–675. doi: 10.1126/science.2823384. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Minette P., Maclagan J. Muscarinic receptor subtypes in airways. Trends Pharmacol Sci. 1988 Nov;9(11):412–416. doi: 10.1016/0165-6147(88)90069-7. [DOI] [PubMed] [Google Scholar]
- Best L., Brooks K. J., Bolton T. B. Relationship between stimulated inositol lipid hydrolysis and contractility in guinea-pig visceral longitudinal smooth muscle. Biochem Pharmacol. 1985 Jul 1;34(13):2297–2301. doi: 10.1016/0006-2952(85)90785-3. [DOI] [PubMed] [Google Scholar]
- Buckley N. J., Bonner T. I., Buckley C. M., Brann M. R. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 1989 Apr;35(4):469–476. [PubMed] [Google Scholar]
- Gardner A. L., Choo L. K., Mitchelson F. Comparison of the effects of some muscarinic agonists on smooth muscle function and phosphatidylinositol turnover in the guinea-pig taenia caeci. Br J Pharmacol. 1988 May;94(1):199–211. doi: 10.1111/j.1476-5381.1988.tb11516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo E., Viganò M. A., Hammer R., Ladinsky H. Characterization of muscarinic receptors in guinea pig ileum longitudinal smooth muscle. Mol Pharmacol. 1988 Jun;33(6):617–625. [PubMed] [Google Scholar]
- Grandordy B. M., Cuss F. M., Sampson A. S., Palmer J. B., Barnes P. J. Phosphatidylinositol response to cholinergic agonists in airway smooth muscle: relationship to contraction and muscarinic receptor occupancy. J Pharmacol Exp Ther. 1986 Jul;238(1):273–279. [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Opposite effects of cyclic GMP and cyclic AMP on Ca2+ current in single heart cells. Nature. 1986 Sep 18;323(6085):273–275. doi: 10.1038/323273a0. [DOI] [PubMed] [Google Scholar]
- Inoue R., Isenberg G. Acetylcholine activates nonselective cation channels in guinea pig ileum through a G protein. Am J Physiol. 1990 Jun;258(6 Pt 1):C1173–C1178. doi: 10.1152/ajpcell.1990.258.6.C1173. [DOI] [PubMed] [Google Scholar]
- Komori K., Suzuki H. Heterogeneous distribution of muscarinic receptors in the rabbit saphenous artery. Br J Pharmacol. 1987 Nov;92(3):657–664. doi: 10.1111/j.1476-5381.1987.tb11369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. P., Kuo J. F., Greengard P. Role of muscarinic cholinergic receptors in regulation of guanosine 3':5'-cyclic monophosphate content in mammalian brain, heart muscle, and intestinal smooth muscle. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3287–3291. doi: 10.1073/pnas.69.11.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchesi P. A., Romano F. D., Scheid C. R., Yamaguchi H., Honeyman T. W. Interaction of agonists and selective antagonists with gastric smooth muscle muscarinic receptors. Naunyn Schmiedebergs Arch Pharmacol. 1989 Jan-Feb;339(1-2):145–151. doi: 10.1007/BF00165136. [DOI] [PubMed] [Google Scholar]
- Moummi C., Magous R., Strosberg D., Bali J. P. Muscarinic receptors in isolated smooth muscle cells from gastric antrum. Biochem Pharmacol. 1988 Apr 1;37(7):1363–1369. doi: 10.1016/0006-2952(88)90795-2. [DOI] [PubMed] [Google Scholar]
- Parekh A. B., Brading A. F. The sources of calcium for carbachol-induced contraction in the circular smooth muscle of guinea-pig stomach. Br J Pharmacol. 1991 Oct;104(2):412–418. doi: 10.1111/j.1476-5381.1991.tb12444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedder E. K., Eveleigh P., Poyner D., Hulme E. C., Birdsall N. J. Modulation of the structure-binding relationships of antagonists for muscarinic acetylcholine receptor subtypes. Br J Pharmacol. 1991 Jun;103(2):1561–1567. doi: 10.1111/j.1476-5381.1991.tb09827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringer M. J., Hyman P. E., Kao H. W., Hsu C. T., Tomomasa T., Snape W. J., Jr [3H]QNB binding and contraction of rabbit colonic smooth muscle cells. Am J Physiol. 1987 Nov;253(5 Pt 1):G656–G661. doi: 10.1152/ajpgi.1987.253.5.G656. [DOI] [PubMed] [Google Scholar]
- Shuttleworth T. J. Receptor-activated calcium entry in exocrine cells does not occur via agonist-sensitive intracellular pools. Biochem J. 1990 Mar 15;266(3):719–726. doi: 10.1042/bj2660719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]
- Zappia L., Cartellà A., Potenzoni D., Bertaccini G. Action of pirenzepine on the human urinary bladder in vitro. J Urol. 1986 Sep;136(3):739–742. doi: 10.1016/s0022-5347(17)45039-7. [DOI] [PubMed] [Google Scholar]


