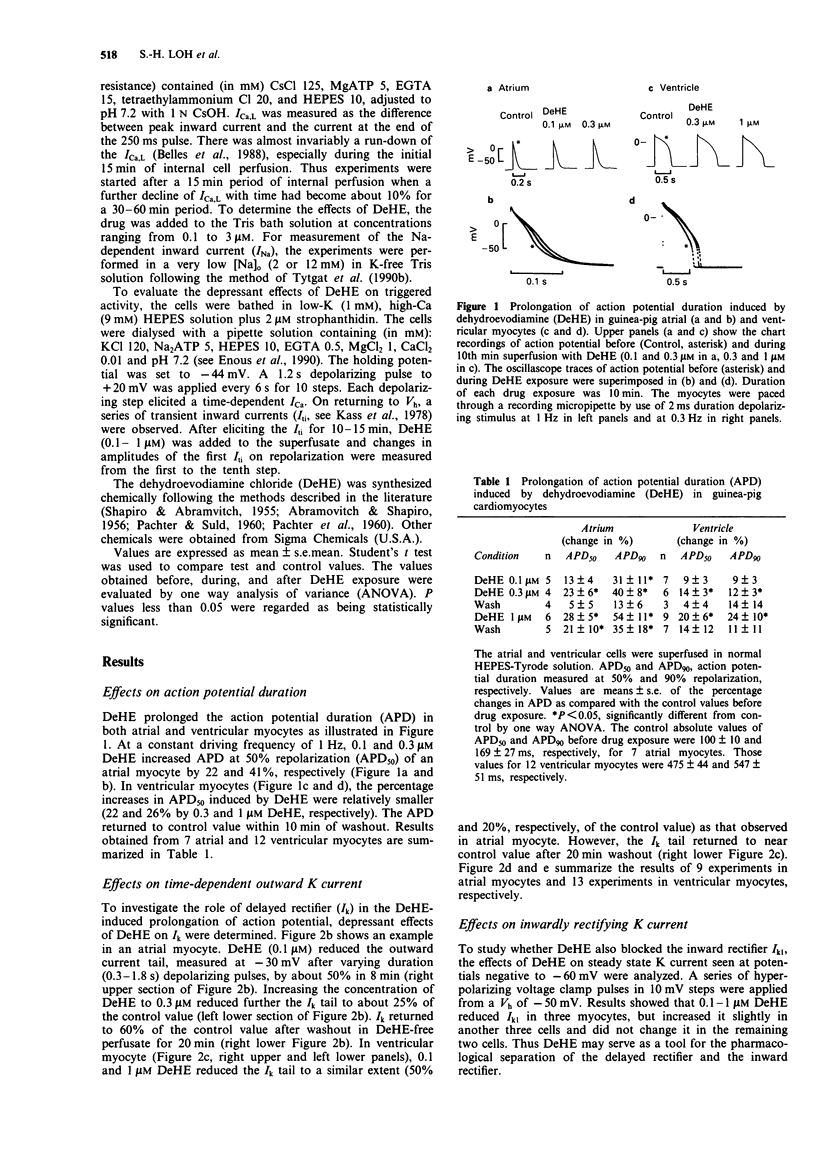
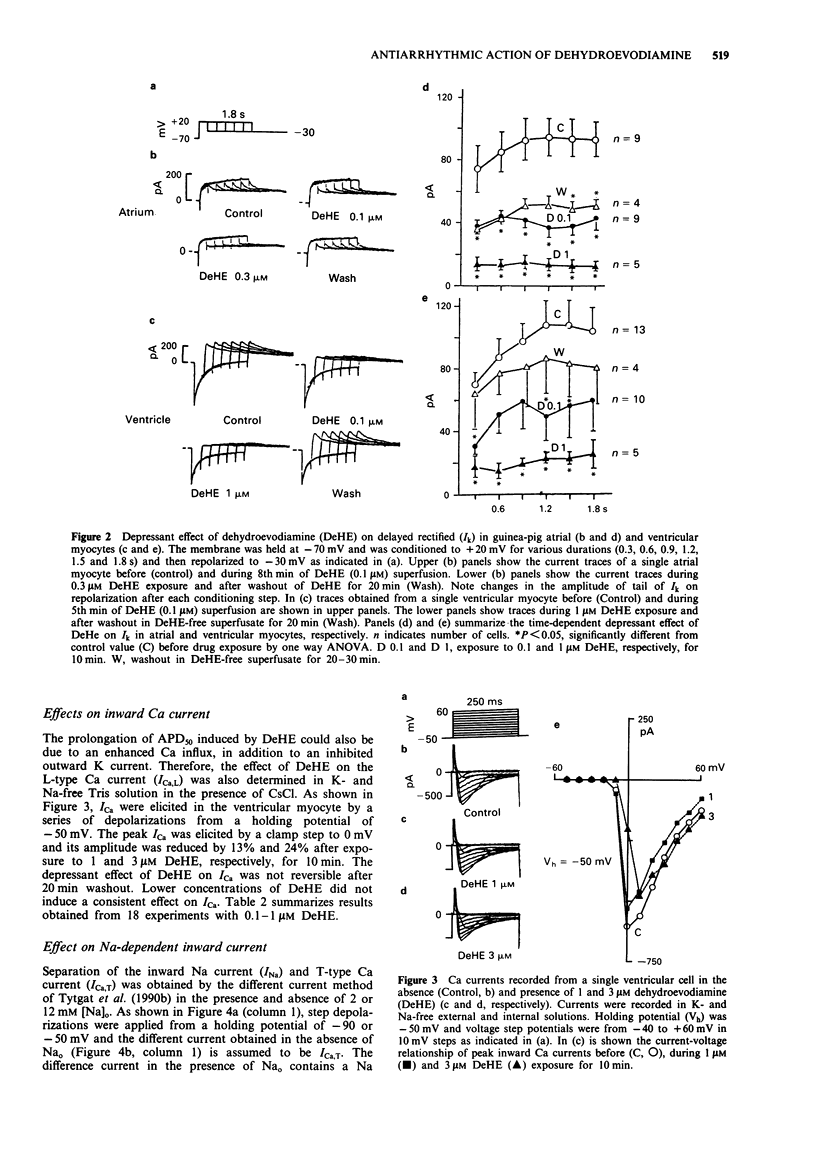
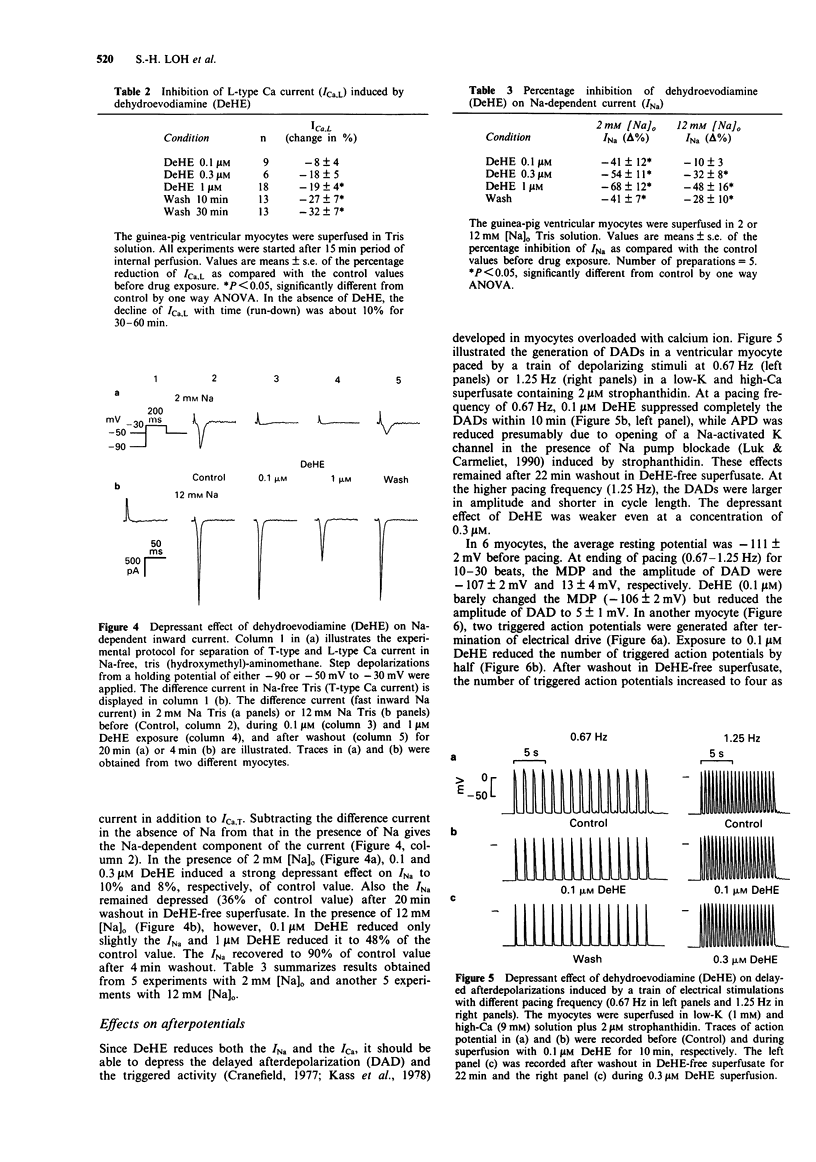
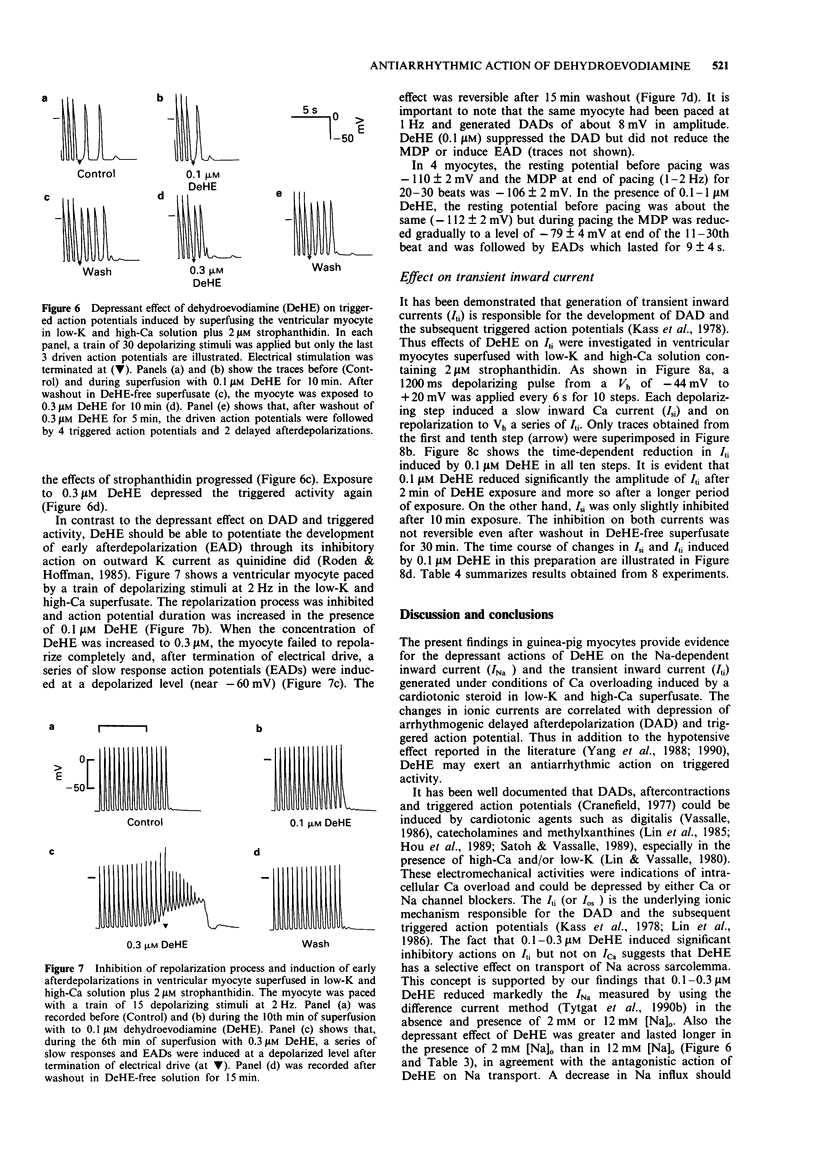
Abstract
1. Dehydroevodiamine alkaloid (DeHE), an active ingredient of a Chinese herbal medicine Wu-Chu-Yu (Evodiae frutus), has been shown to decrease aterial blood pressure in experimental animals and prolong action potential duration in cardiac cells. The aim of the present study was to explore the ionic basis of its possible antiarrhythmic effects. 2. Guinea-pig atrial and ventricular myocytes were isolated enzymatically and the ionic currents were recorded under whole-cell patch-clamp with single suction pipettes. 3. DeHE at a concentration of 0.1 microM inhibited reversibly the time-dependent outward K current (delayed rectifier, Ik) and the Na-dependent inward current (INa). 4. In low-K (1 mM) and high-Ca (9 mM) solution, DeHE also depressed the delayed afterdepolarizations (DAD) and the transient inward current (Iti) induced by 2 microM strophanthidin. On the other hand, DeHE occasionally induced early afterdepolarizations and slow response action potentials at a depolarized level. 5. At higher concentrations (1 microM and above), the L-type Ca current (ICa,L) was moderately inhibited. 6. The present findings indicate that DeHE may depress triggered arrhythmias in Ca-overloaded guinea-pig cardiac myocytes through its inhibitory actions on INa, Iti and, to a smaller extent, ICa. DeHE may also exert class III antiarrhythmic effect through a reduction of outward K currents (Ik) across the sarcolemma.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belles B., Malécot C. O., Hescheler J., Trautwein W. "Run-down" of the Ca current during long whole-cell recordings in guinea pig heart cells: role of phosphorylation and intracellular calcium. Pflugers Arch. 1988 Apr;411(4):353–360. doi: 10.1007/BF00587713. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Sodium-calcium exchange in guinea-pig cardiac cells: exchange current and changes in intracellular Ca2+. J Physiol. 1989 Jul;414:499–520. doi: 10.1113/jphysiol.1989.sp017700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. F., Chen S. M., Lin M. T., Chow S. Y. In vivo and in vitro studies on the mechanism of cardiovascular effects of Wu-Chu-Yu (Evodiae fructus). Am J Chin Med. 1981 Spring;9(1):39–47. doi: 10.1142/s0192415x81000068. [DOI] [PubMed] [Google Scholar]
- Cranefield P. F. Action potentials, afterpotentials, and arrhythmias. Circ Res. 1977 Oct;41(4):415–423. doi: 10.1161/01.res.41.4.415. [DOI] [PubMed] [Google Scholar]
- Hou Z. Y., Lin C. I., Vassalle M., Chiang B. N., Cheng K. K. Role of acetylcholine in induction of repetitive activity in human atrial fibers. Am J Physiol. 1989 Jan;256(1 Pt 2):H74–H84. doi: 10.1152/ajpheart.1989.256.1.H74. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987 Mar;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. I., Chiu T. H., Chiang B. N., Cheng K. K. Electromechanical effects of caffeine in isolated human atrial fibres. Cardiovasc Res. 1985 Dec;19(12):727–733. doi: 10.1093/cvr/19.12.727. [DOI] [PubMed] [Google Scholar]
- Lin C. I., Kotake H., Vassalle M. On the mechanism underlying the oscillatory current in cardiac Purkinje fibers. J Cardiovasc Pharmacol. 1986 Sep-Oct;8(5):906–914. doi: 10.1097/00005344-198609000-00004. [DOI] [PubMed] [Google Scholar]
- Lin C. I., Vassalle M. The antiarrhythmic effect of potassium and rubidium in strophanthidin toxicity. Eur J Pharmacol. 1980 Mar 7;62(1):1–15. doi: 10.1016/0014-2999(80)90475-6. [DOI] [PubMed] [Google Scholar]
- Luk H. N., Carmeliet E. Na(+)-activated K+ current in cardiac cells: rectification, open probability, block and role in digitalis toxicity. Pflugers Arch. 1990 Aug;416(6):766–768. doi: 10.1007/BF00370627. [DOI] [PubMed] [Google Scholar]
- Mitra R., Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985 Nov;249(5 Pt 2):H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- Roden D. M., Hoffman B. F. Action potential prolongation and induction of abnormal automaticity by low quinidine concentrations in canine Purkinje fibers. Relationship to potassium and cycle length. Circ Res. 1985 Jun;56(6):857–867. doi: 10.1161/01.res.56.6.857. [DOI] [PubMed] [Google Scholar]
- Satoh H., Vassalle M. Role of calcium in caffeine-norepinephrine interactions in cardiac Purkinje fibers. Am J Physiol. 1989 Jul;257(1 Pt 2):H226–H237. doi: 10.1152/ajpheart.1989.257.1.H226. [DOI] [PubMed] [Google Scholar]
- Singh B. N., Nademanee K. Control of cardiac arrhythmias by selective lengthening of repolarization: theoretic considerations and clinical observations. Am Heart J. 1985 Feb;109(2):421–430. doi: 10.1016/0002-8703(85)90629-5. [DOI] [PubMed] [Google Scholar]
- Tytgat J., Vereecke J., Carmeliet E. A combined study of sodium current and T-type calcium current in isolated cardiac cells. Pflugers Arch. 1990 Oct;417(2):142–148. doi: 10.1007/BF00370691. [DOI] [PubMed] [Google Scholar]
- Tytgat J., Vereecke J., Carmeliet E. Mechanism of cardiac T-type Ca channel blockade by amiloride. J Pharmacol Exp Ther. 1990 Aug;254(2):546–551. [PubMed] [Google Scholar]
- Vassalle M., Lee C. O. The relationship among intracellular sodium activity, calcium, and strophanthidin inotropy in canine cardiac Purkinje fibers. J Gen Physiol. 1984 Feb;83(2):287–307. doi: 10.1085/jgp.83.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. C., Wu S. L., Kuo J. S., Chen C. F. The hypotensive and negative chronotropic effects of dehydroevodiamine. Eur J Pharmacol. 1990 Jul 17;182(3):537–542. doi: 10.1016/0014-2999(90)90052-8. [DOI] [PubMed] [Google Scholar]


