Abstract
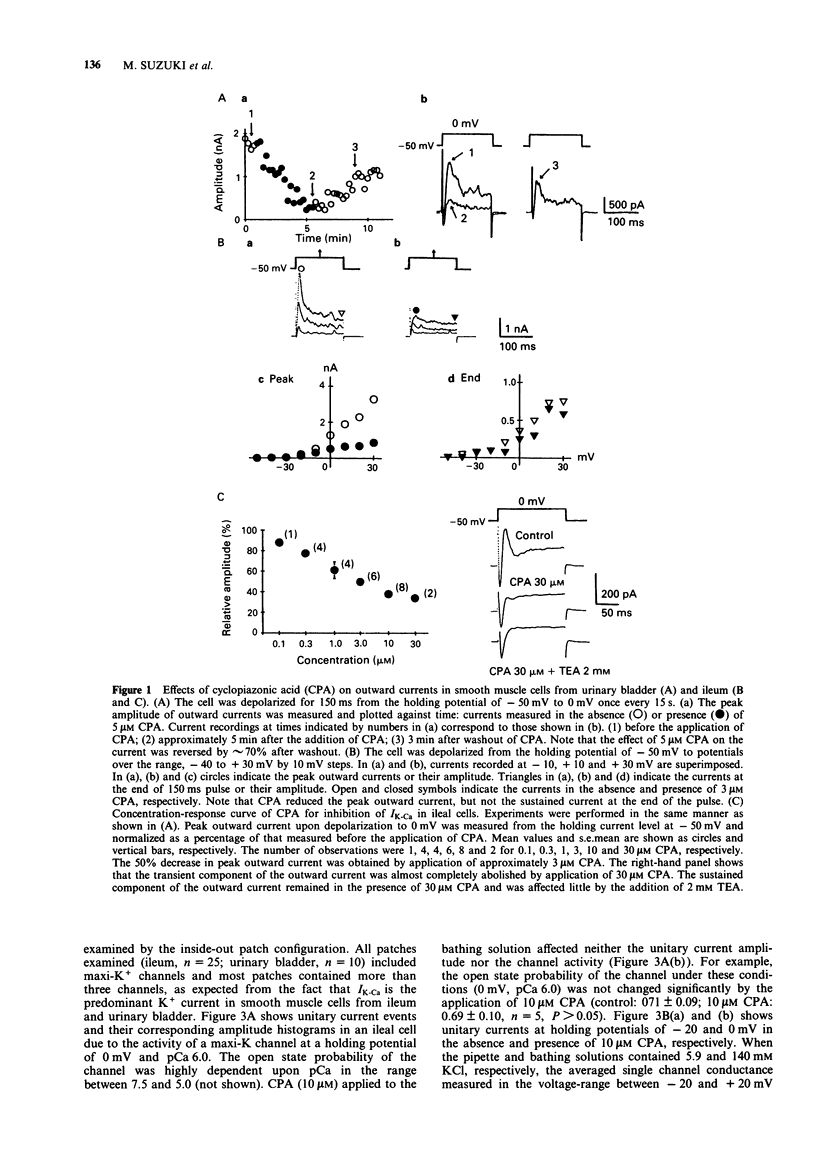
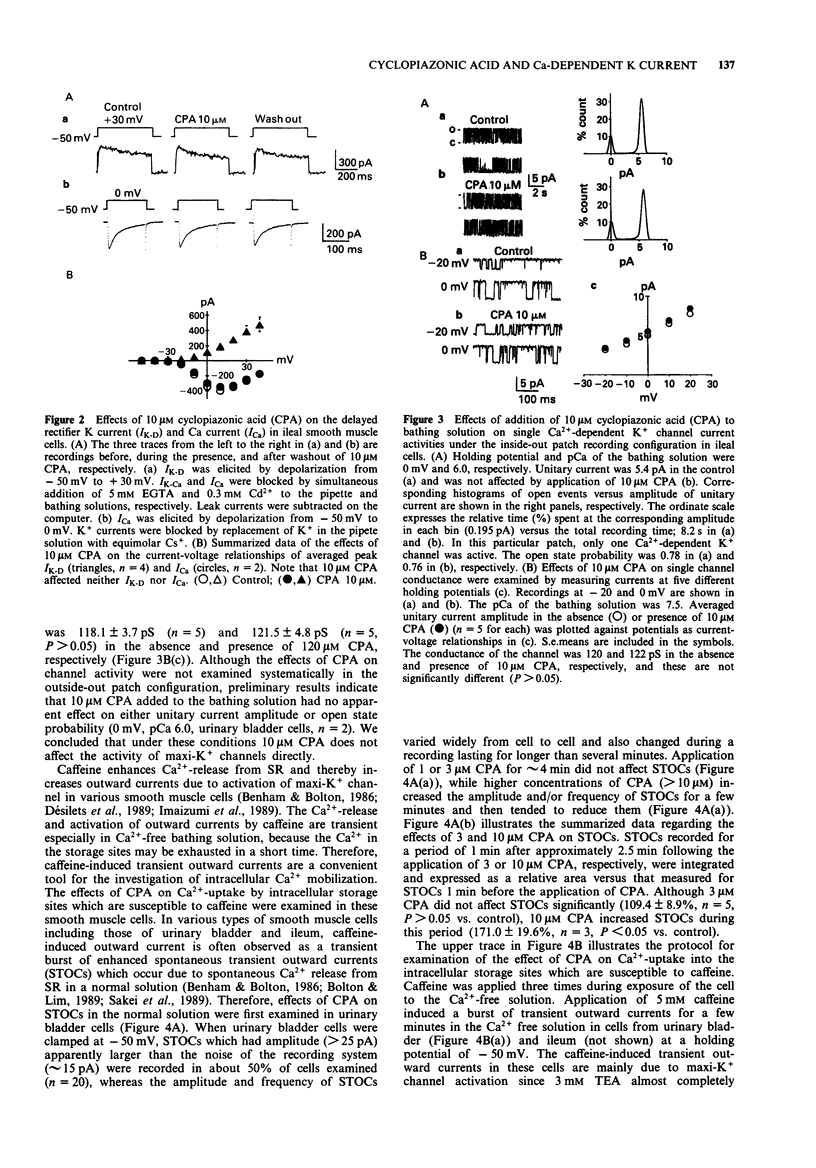
1. Effects of cyclopiazonic acid (CPA), a specific inhibitor of the Ca(2+)-ATPase in sarcoplasmic reticulum (SR), on membrane ionic currents were examined in single smooth muscle cells freshly isolated from ileal longitudinal strips and urinary bladder of the guinea-pig. 2. Under whole-cell clamp, CPA (1-10 microM) reduced peak outward current elicited by depolarization in a concentration-dependent manner. The concentration of CPA required for 50% decrease in the peak outward current was approximately 3 microM in ileal cells under these conditions. The current reduced by CPA recovered by more than 70% after washout. 3. The transient outward current elicited by application of 5 mM caffeine at a holding potential of -50 mV in Ca2+ free solution was almost abolished, when the preceding Ca(2+)-loading of the cell in a solution containing 2.2 mM Ca2+ was performed in the presence of 3 microM CPA. 4. When the Ca(2+)-dependent K+ current (IK-Ca) and Ca2+ current (ICa) were inhibited by addition of Ca2+, the remaining delayed rectifier type K+ current was not affected by 10 microM CPA. When outward currents were blocked by replacement of K+ by Cs+ in the pipette solution, the remaining ICa was not affected by 10 microM CPA. 5. CPA (10 microM) did not affect the conductance of single maxi Ca(2+)-dependent K+ channels or the Cd(2+)-dependence of their open probability in both inside- and outside-out configurations. 6. These results indicate that IK-Ca is selectively and strongly suppressed by CPA.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amédée T., Large W. A., Wang Q. Characteristics of chloride currents activated by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Sep;428:501–516. doi: 10.1113/jphysiol.1990.sp018224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld D. R., Hume J. R., Krier J. Action potentials and membrane currents of isolated single smooth muscle cells of cat and rabbit colon. Pflugers Arch. 1990 Mar;415(6):678–687. doi: 10.1007/BF02584005. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Lim S. P. Properties of calcium stores and transient outward currents in single smooth muscle cells of rabbit intestine. J Physiol. 1989 Feb;409:385–401. doi: 10.1113/jphysiol.1989.sp017504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourreau J. P., Abela A. P., Kwan C. Y., Daniel E. E. Acetylcholine Ca2+ stores refilling directly involves a dihydropyridine-sensitive channel in dog trachea. Am J Physiol. 1991 Sep;261(3 Pt 1):C497–C505. doi: 10.1152/ajpcell.1991.261.3.C497. [DOI] [PubMed] [Google Scholar]
- Carl A., McHale N. G., Publicover N. G., Sanders K. M. Participation of Ca2(+)-activated K+ channels in electrical activity of canine gastric smooth muscle. J Physiol. 1990 Oct;429:205–221. doi: 10.1113/jphysiol.1990.sp018252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole W. C., Carl A., Sanders K. M. Muscarinic suppression of Ca2+-dependent K current in colonic smooth muscle. Am J Physiol. 1989 Sep;257(3 Pt 1):C481–C487. doi: 10.1152/ajpcell.1989.257.3.C481. [DOI] [PubMed] [Google Scholar]
- Cole W. C., Sanders K. M. Characterization of macroscopic outward currents of canine colonic myocytes. Am J Physiol. 1989 Sep;257(3 Pt 1):C461–C469. doi: 10.1152/ajpcell.1989.257.3.C461. [DOI] [PubMed] [Google Scholar]
- Deng H. W., Kwan C. Y. Cyclopiazonic acid is a sarcoplasmic reticulum Ca(2+)-pump inhibitor of rat aortic muscle. Zhongguo Yao Li Xue Bao. 1991 Jan;12(1):53–58. [PubMed] [Google Scholar]
- Désilets M., Driska S. P., Baumgarten C. M. Current fluctuations and oscillations in smooth muscle cells from hog carotid artery. Role of the sarcoplasmic reticulum. Circ Res. 1989 Sep;65(3):708–722. doi: 10.1161/01.res.65.3.708. [DOI] [PubMed] [Google Scholar]
- Ganitkevich V., Isenberg G. Isolated guinea pig coronary smooth muscle cells. Acetylcholine induces hyperpolarization due to sarcoplasmic reticulum calcium release activating potassium channels. Circ Res. 1990 Aug;67(2):525–528. doi: 10.1161/01.res.67.2.525. [DOI] [PubMed] [Google Scholar]
- Goeger D. E., Riley R. T., Dorner J. W., Cole R. J. Cyclopiazonic acid inhibition of the Ca2+-transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles. Biochem Pharmacol. 1988 Mar 1;37(5):978–981. doi: 10.1016/0006-2952(88)90195-5. [DOI] [PubMed] [Google Scholar]
- Goeger D. E., Riley R. T. Interaction of cyclopiazonic acid with rat skeletal muscle sarcoplasmic reticulum vesicles. Effect on Ca2+ binding and Ca2+ permeability. Biochem Pharmacol. 1989 Nov 15;38(22):3995–4003. doi: 10.1016/0006-2952(89)90679-5. [DOI] [PubMed] [Google Scholar]
- Green K. A., Foster R. W., Small R. C. A patch-clamp study of K(+)-channel activity in bovine isolated tracheal smooth muscle cells. Br J Pharmacol. 1991 Apr;102(4):871–878. doi: 10.1111/j.1476-5381.1991.tb12269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Iino M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 1989 Aug;94(2):363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Muraki K., Watanabe M. Characteristics of transient outward currents in single smooth muscle cells from the ureter of the guinea-pig. J Physiol. 1990 Aug;427:301–324. doi: 10.1113/jphysiol.1990.sp018173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Muraki K., Watanabe M. Ionic currents in single smooth muscle cells from the ureter of the guinea-pig. J Physiol. 1989 Apr;411:131–159. doi: 10.1113/jphysiol.1989.sp017565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Two Ca-dependent K-channels classified by the application of tetraethylammonium distribute to smooth muscle membranes of the rabbit portal vein. Pflugers Arch. 1985 Oct;405(3):173–179. doi: 10.1007/BF00582557. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Sakai T., Kajioka S., Kuriyama H. Activations of the Ca dependent K channel by Ca released from the sarcoplasmic reticulum of mammalian smooth muscles. Biomed Biochim Acta. 1989;48(5-6):S364–S369. [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 1985 Dec;405(4):329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Calcium release induced by inositol 1,4,5-trisphosphate in single rabbit intestinal smooth muscle cells. J Physiol. 1991 Feb;433:495–517. doi: 10.1113/jphysiol.1991.sp018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Role of G-proteins in muscarinic receptor inward and outward currents in rabbit jejunal smooth muscle. J Physiol. 1990 Aug;427:395–419. doi: 10.1113/jphysiol.1990.sp018178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi N., Ogawa Y. Discrimination of Ca(2+)-ATPase activity of the sarcoplasmic reticulum from actomyosin-type ATPase activity of myofibrils in skinned mammalian skeletal muscle fibres: distinct effects of cyclopiazonic acid on the two ATPase activities. J Muscle Res Cell Motil. 1991 Aug;12(4):355–365. doi: 10.1007/BF01738590. [DOI] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Loirand G., Pacaud P., Baron A., Mironneau C., Mironneau J. Large conductance calcium-activated non-selective cation channel in smooth muscle cells isolated from rat portal vein. J Physiol. 1991 Jun;437:461–475. doi: 10.1113/jphysiol.1991.sp018606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987 Apr;252(4 Pt 1):C401–C410. doi: 10.1152/ajpcell.1987.252.4.C401. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Terada K., Kitamura K., Kuriyama H. Membrane currents recorded from a fragment of rabbit intestinal smooth muscle cell. Am J Physiol. 1986 Sep;251(3 Pt 1):C335–C346. doi: 10.1152/ajpcell.1986.251.3.C335. [DOI] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Lavie J. L., Mironneau C., Mironneau J. Calcium-activated chloride current in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1989 Apr;413(6):629–636. doi: 10.1007/BF00581813. [DOI] [PubMed] [Google Scholar]
- Sakai T., Terada K., Kitamura K., Kuriyama H. Ryanodine inhibits the Ca-dependent K current after depletion of Ca stored in smooth muscle cells of the rabbit ileal longitudinal muscle. Br J Pharmacol. 1988 Dec;95(4):1089–1100. doi: 10.1111/j.1476-5381.1988.tb11743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler N. W., Jona I., Vegh M., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989 Oct 25;264(30):17816–17823. [PubMed] [Google Scholar]
- Sims S. M., Vivaudou M. B., Hillemeier C., Biancani P., Walsh J. V., Jr, Singer J. J. Membrane currents and cholinergic regulation of K+ current in esophageal smooth muscle cells. Am J Physiol. 1990 May;258(5 Pt 1):G794–G802. doi: 10.1152/ajpgi.1990.258.5.G794. [DOI] [PubMed] [Google Scholar]
- Takeda M., Imaizumi Y., Watanabe M. Effects of noradrenaline and heparin on outward current in single smooth muscle cells of the guinea-pig vas deferens. Eur J Pharmacol. 1991 Feb 14;193(3):375–378. doi: 10.1016/0014-2999(91)90155-j. [DOI] [PubMed] [Google Scholar]
- Terada K., Kitamura K., Kuriyama H. Different inhibitions of the voltage-dependent K+ current by Ca2+ antagonists in the smooth muscle cell membrane of rabbit small intestine. Pflugers Arch. 1987 May;408(6):558–564. doi: 10.1007/BF00581156. [DOI] [PubMed] [Google Scholar]
- Uyama Y., Imaizumi Y., Watanabe M. Effects of cyclopiazonic acid, a novel Ca(2+)-ATPase inhibitor, on contractile responses in skinned ileal smooth muscle. Br J Pharmacol. 1992 May;106(1):208–214. doi: 10.1111/j.1476-5381.1992.tb14316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogalis F., Publicover N. G., Hume J. R., Sanders K. M. Relationship between calcium current and cytosolic calcium in canine gastric smooth muscle cells. Am J Physiol. 1991 May;260(5 Pt 1):C1012–C1018. doi: 10.1152/ajpcell.1991.260.5.C1012. [DOI] [PubMed] [Google Scholar]
- Wang Q., Large W. A. Noradrenaline-evoked cation conductance recorded with the nystatin whole-cell method in rabbit portal vein cells. J Physiol. 1991 Apr;435:21–39. doi: 10.1113/jphysiol.1991.sp018496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Imaizumi Y., Muraki K., Takeda M. A comparative study about voltage-dependent Ca currents in smooth muscle cells isolated from several tissues. Adv Exp Med Biol. 1989;255:119–128. doi: 10.1007/978-1-4684-5679-0_13. [DOI] [PubMed] [Google Scholar]


