Abstract
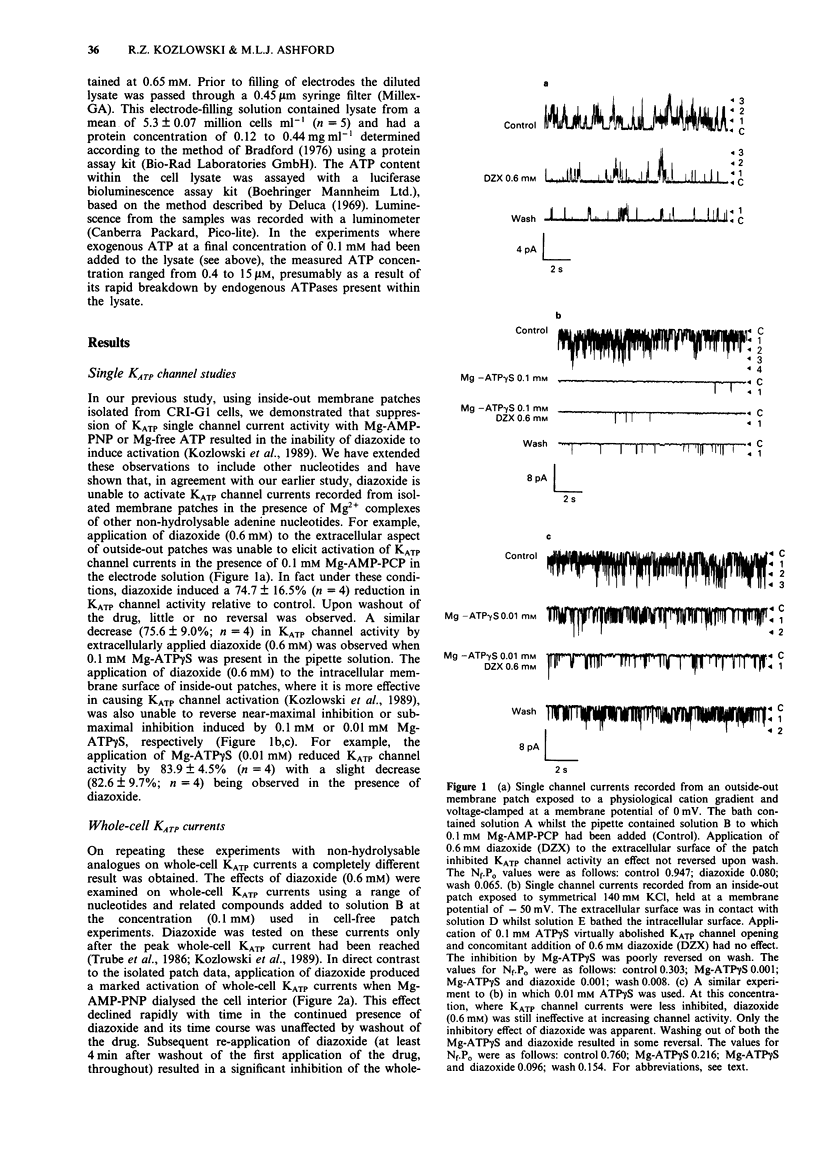
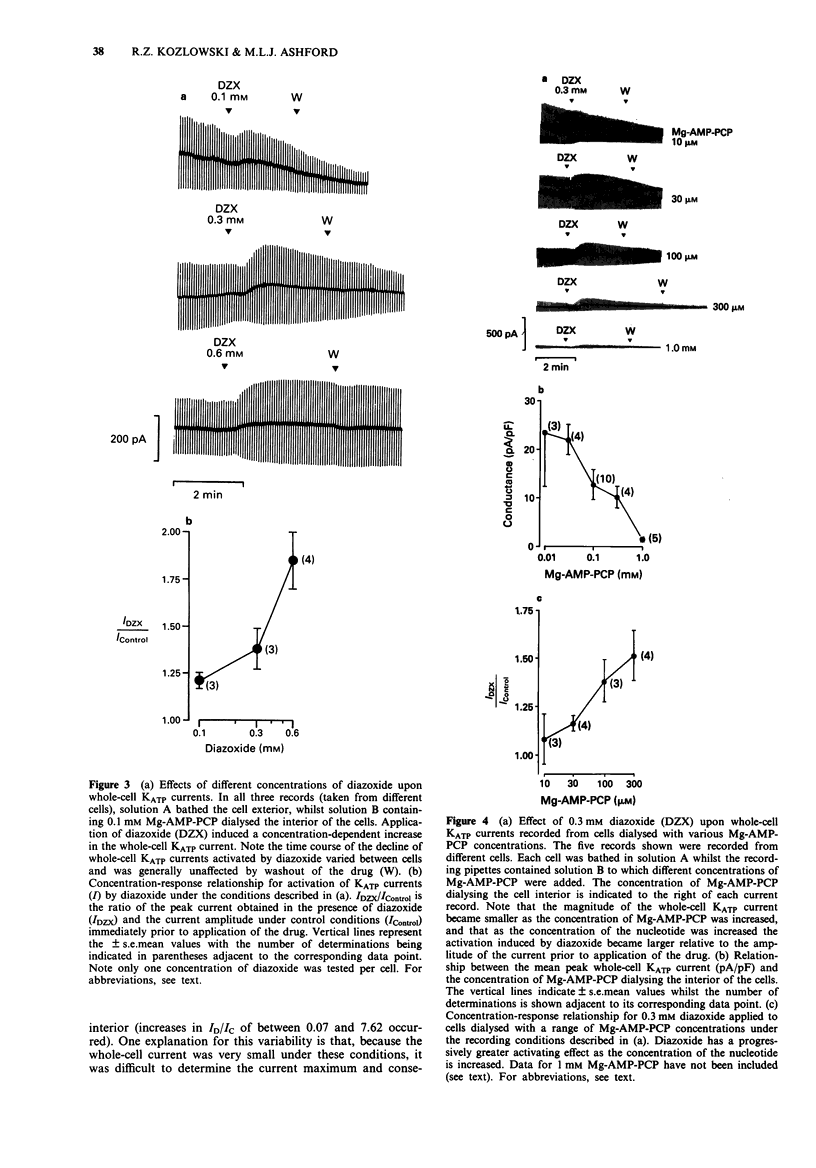
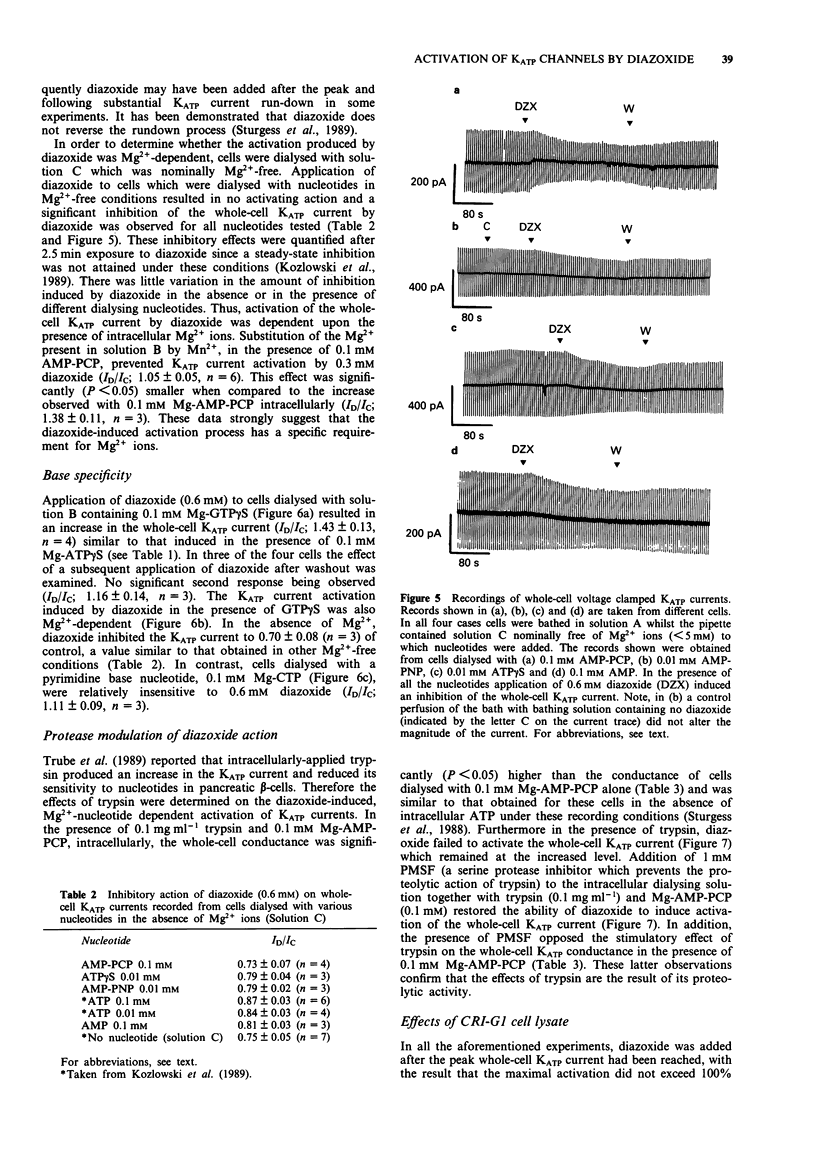
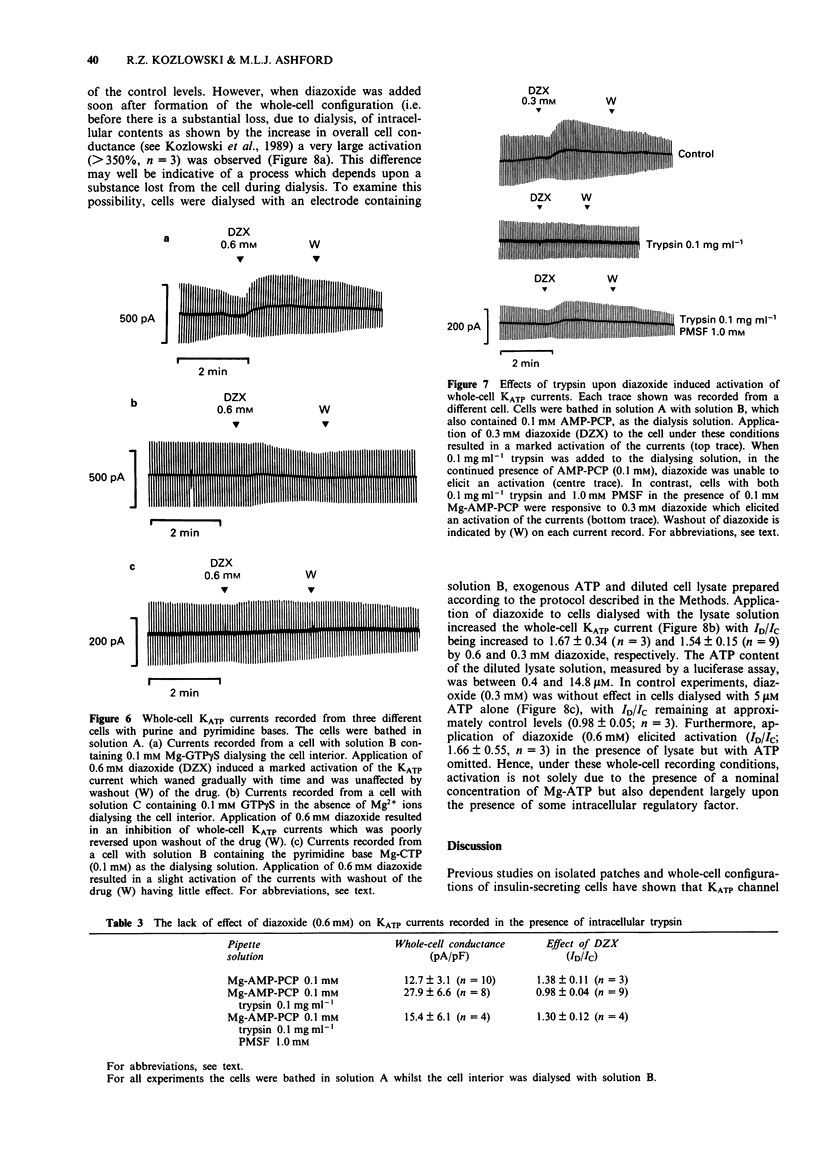
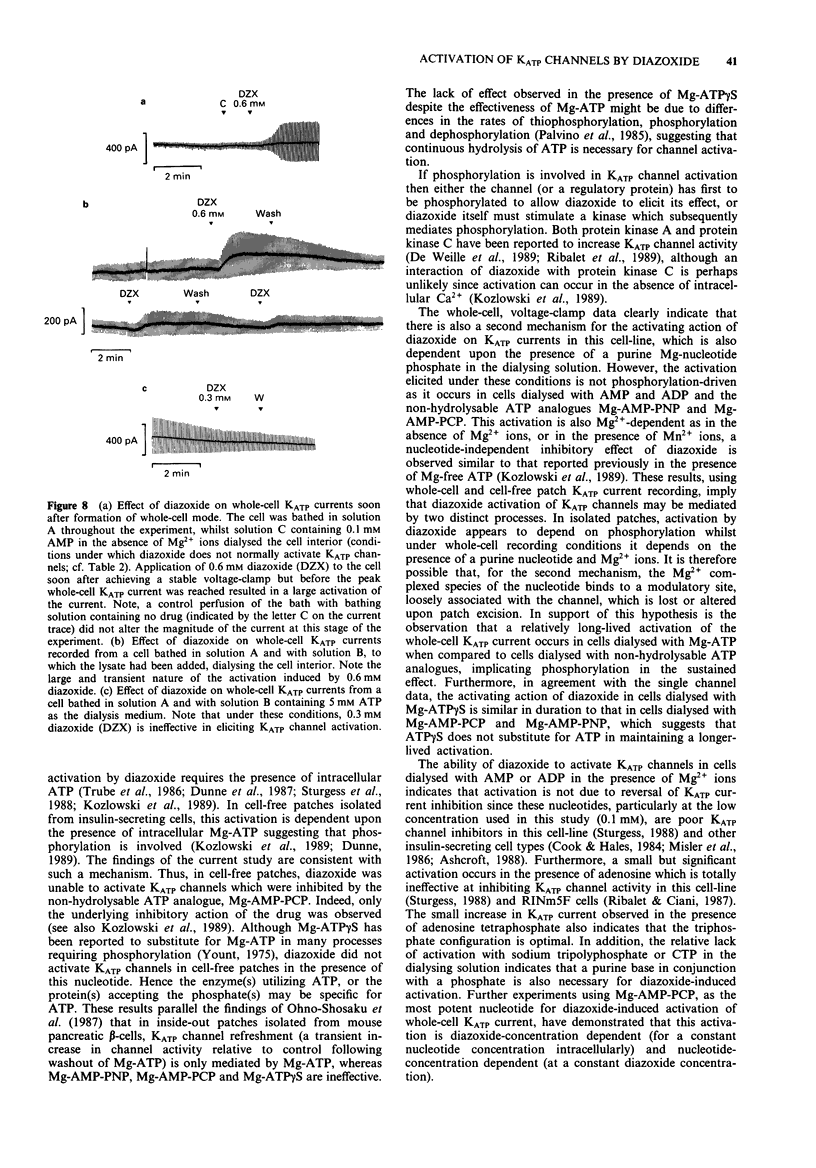
1. Patch-clamp recording techniques were used, to examine the effects of diazoxide on KATP currents in CRI-G1 insulin-secreting cells in the presence of non-hydrolysable nucleotides. 2. In the presence of non- or slowly-hydrolyzed ATP analogues, bathing the intracellular aspect of cell-free membrane patches diazoxide inhibited KATP channel activity. 3. Under whole-cell recording conditions, with various non-hydrolysable nucleotides present intracellularly (after dialysis), diazoxide induced KATP current activation. The largest activation occurred with Mg-adenylyl-(beta, gamma-methylene) diphosphate (Mg-AMP-PCP) present in the dialysing solution. This activation was diazoxide- and nucleotide-concentration-dependent. 4. In the absence of Mg2+, or in the presence of manganese (Mn2+) ions intracellularly, diazoxide did not induce KATP current activation, regardless of the species of nucleotide present in the pipette. 5. Intracellularly applied trypsin prevented the activation of KATP currents by diazoxide in the presence of Mg-AMP-PCP, an effect reversed by co-application of intracellular polymethylsulphonyl fluoride with the trypsin. 6. The application, by dialysis, of a CRI-G1 cell lysate, with negligible Mg-ATP, resulted in a substantial activation of the KATP current by diazoxide. 7. It is concluded that diazoxide can activate KATP channel currents by two separate pathways, one requiring a phosphorylation process, the other the presence of an intracellular protein coupled with a Mg-purine nucleotide.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Ashcroft S. J., Harrison D. E. Effects of 2-ketoisocaproate on insulin release and single potassium channel activity in dispersed rat pancreatic beta-cells. J Physiol. 1987 Apr;385:517–529. doi: 10.1113/jphysiol.1987.sp016505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Ashcroft F. M. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2(3):197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carrington C. A., Rubery E. D., Pearson E. C., Hales C. N. Five new insulin-producing cell lines with differing secretory properties. J Endocrinol. 1986 May;109(2):193–200. doi: 10.1677/joe.0.1090193. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Deluca M. Firefly luciferase. Adv Enzymol Relat Areas Mol Biol. 1976;44:37–68. doi: 10.1002/9780470122891.ch2. [DOI] [PubMed] [Google Scholar]
- Dunne M. J. Protein phosphorylation is required for diazoxide to open ATP-sensitive potassium channels in insulin (RINm5F) secreting cells. FEBS Lett. 1989 Jul 3;250(2):262–266. doi: 10.1016/0014-5793(89)80734-3. [DOI] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. Structure-activity relationships of K+ channel openers. Trends Pharmacol Sci. 1990 Oct;11(10):417–422. doi: 10.1016/0165-6147(90)90149-3. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochem Pharmacol. 1982 Apr 1;31(7):1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Kozlowski R. Z., Ashford M. L. ATP-sensitive K(+)-channel run-down is Mg2+ dependent. Proc R Soc Lond B Biol Sci. 1990 Jun 22;240(1298):397–410. doi: 10.1098/rspb.1990.0044. [DOI] [PubMed] [Google Scholar]
- Kozlowski R. Z., Hales C. N., Ashford M. L. Dual effects of diazoxide on ATP-K+ currents recorded from an insulin-secreting cell line. Br J Pharmacol. 1989 Aug;97(4):1039–1050. doi: 10.1111/j.1476-5381.1989.tb12560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misler S., Falke L. C., Gillis K., McDaniel M. L. A metabolite-regulated potassium channel in rat pancreatic B cells. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7119–7123. doi: 10.1073/pnas.83.18.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Zünkler B. J., Trube G. Dual effects of ATP on K+ currents of mouse pancreatic beta-cells. Pflugers Arch. 1987 Feb;408(2):133–138. doi: 10.1007/BF00581342. [DOI] [PubMed] [Google Scholar]
- Palvimo J., Linnala-Kankkunen A., Mäenpä P. H. Thiophosphorylation and phosphorylation of chromatin proteins from calf thymus in vitro. Biochem Biophys Res Commun. 1985 Jan 16;126(1):103–108. doi: 10.1016/0006-291x(85)90577-7. [DOI] [PubMed] [Google Scholar]
- Quast U., Cook N. S. Moving together: K+ channel openers and ATP-sensitive K+ channels. Trends Pharmacol Sci. 1989 Nov;10(11):431–435. doi: 10.1016/S0165-6147(89)80003-3. [DOI] [PubMed] [Google Scholar]
- Ribalet B., Ciani S., Eddlestone G. T. ATP mediates both activation and inhibition of K(ATP) channel activity via cAMP-dependent protein kinase in insulin-secreting cell lines. J Gen Physiol. 1989 Oct;94(4):693–717. doi: 10.1085/jgp.94.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribalet B., Ciani S. Regulation by cell metabolism and adenine nucleotides of a K channel in insulin-secreting B cells (RIN m5F). Proc Natl Acad Sci U S A. 1987 Mar;84(6):1721–1725. doi: 10.1073/pnas.84.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgess N. C., Ashford M. L., Cook D. L., Hales C. N. The sulphonylurea receptor may be an ATP-sensitive potassium channel. Lancet. 1985 Aug 31;2(8453):474–475. doi: 10.1016/s0140-6736(85)90403-9. [DOI] [PubMed] [Google Scholar]
- Sturgess N. C., Kozlowski R. Z., Carrington C. A., Hales C. N., Ashford M. L. Effects of sulphonylureas and diazoxide on insulin secretion and nucleotide-sensitive channels in an insulin-secreting cell line. Br J Pharmacol. 1988 Sep;95(1):83–94. doi: 10.1111/j.1476-5381.1988.tb16551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Yount R. G. ATP analogs. Adv Enzymol Relat Areas Mol Biol. 1975;43:1–56. doi: 10.1002/9780470122884.ch1. [DOI] [PubMed] [Google Scholar]


