Abstract
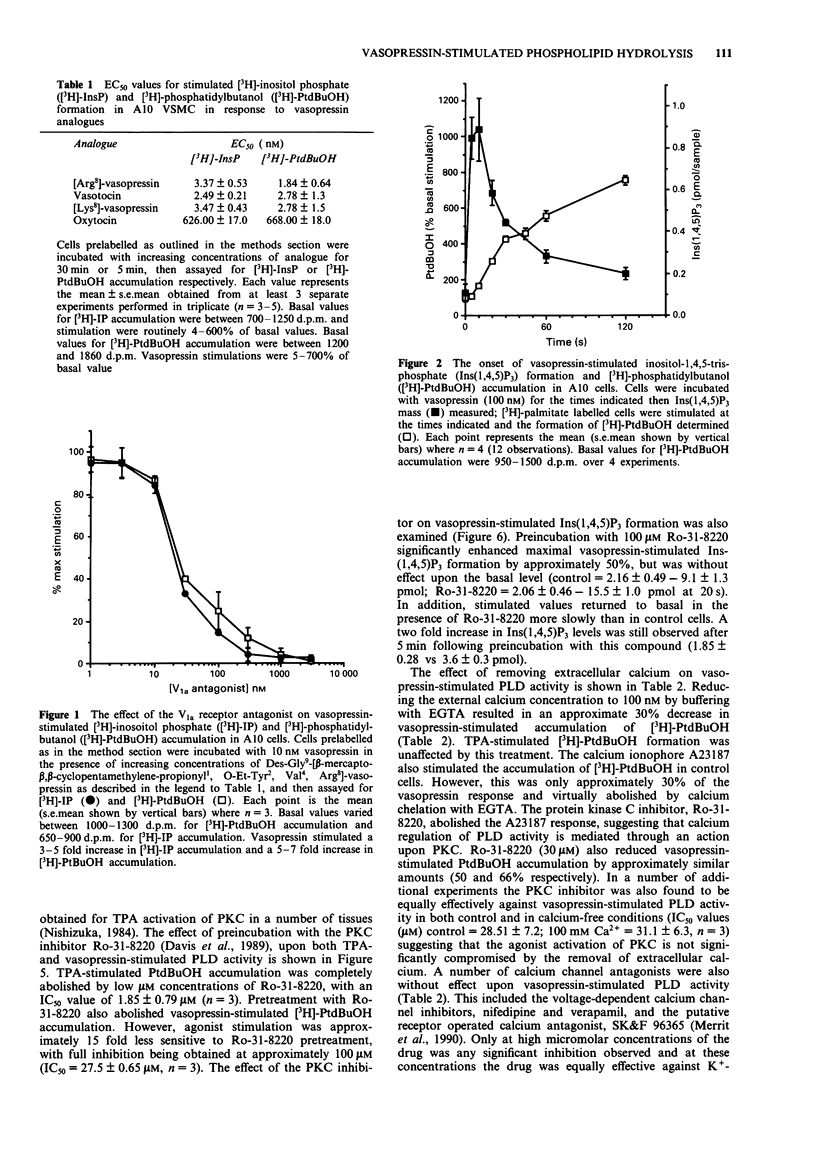
1. The characteristics of vasopressin-stimulated phosphatidylinositol 4,5 bisphosphate (PtdIns(4,5)P2) and phosphatidylcholine (PtdCh) hydrolysis were examined in A10 vascular smooth muscle cells (VSMC), by assessing the formation of [3H]-inositol phosphates ([3H]-IP) and the accumulation of the phospholipase D (PLD) specific product, [3H]-phosphatidylbutanol ([3H]-PtdBuOH). 2. Vasopressin ([Arg8]-VP) and a number of related analogues stimulated the accumulation of [3H]-IP and [3H]-PtdBuOH with similar EC50 values, generating the same rank order of potency for each response (Arg8-VP = vasotocin = Lys8-VP much greater than oxytocin). 3. Inhibition of vasopressin-stimulated [3H]-IP and [3H]-PtdBuOH accumulation by the V1a receptor antagonists, Des-Gly9[beta-mercapto-beta,beta,-cyclopentamethylene propionyl, O-Et-Tyr2,Val4,Arg8]-vasopressin generated similar IC50 values suggesting that both these responses are mediated through the activation of a single V1a receptor subtype. 4. The onset of vasopressin-stimulated inositol-1,4,5-trisphosphate (Ins(1,4,5)P3) mass formation preceded [3H]-PtdBuOH accumulation indicating that PtdCh hydrolysis was activated subsequent to PtdIns(4,5)P2 breakdown. 5. The protein kinase C (PKC) activator, tetradecanoylphorbol acetate (TPA) also stimulated [3H]-PtdBuOH accumulation. Preincubation with the PKC inhibitor Ro-31-8220 abolished both TPA- and vasopressin-stimulated [3H]-PtdBuOH, suggesting that the intermediate activation of protein kinase C is involved in the regulation of PLD by vasopressin. 6. Pretreatment of the A10 VSMC with Ro-31-8220 (100 microM) also potentiated vasopressin-stimulated Ins(1,4,5)P3 mass formation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar N., Nambi P., Stassen F. L., Crooke S. T. Vascular vasopressin receptors mediate phosphatidylinositol turnover and calcium efflux in an established smooth muscle cell line. Life Sci. 1986 Jul 7;39(1):37–45. doi: 10.1016/0024-3205(86)90435-2. [DOI] [PubMed] [Google Scholar]
- Augert G., Bocckino S. B., Blackmore P. F., Exton J. H. Hormonal stimulation of diacylglycerol formation in hepatocytes. Evidence for phosphatidylcholine breakdown. J Biol Chem. 1989 Dec 25;264(36):21689–21698. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Pai J. K., Mullmann T. J., Egan R. W., Siegel M. I. Regulation of phospholipase D in HL-60 granulocytes. Activation by phorbol esters, diglyceride, and calcium ionophore via protein kinase- independent mechanisms. J Biol Chem. 1989 May 25;264(15):9069–9076. [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Wilson P. B., Exton J. H. Phosphatidate accumulation in hormone-treated hepatocytes via a phospholipase D mechanism. J Biol Chem. 1987 Nov 5;262(31):15309–15315. [PubMed] [Google Scholar]
- Brown K. D., Littlewood C. J., Blakeley D. M. Differential potentiation of mitogen-stimulated phosphoinositide hydrolysis in protein kinase C-depleted Swiss 3T3 cells. Biochem J. 1990 Sep 1;270(2):557–560. doi: 10.1042/bj2700557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield M. P., Brown D. A. Pharmacology of the putative M4 muscarinic receptor mediating Ca-current inhibition in neuroblastoma x glioma hybrid (NG 108-15) cells. Br J Pharmacol. 1991 Sep;104(1):39–44. doi: 10.1111/j.1476-5381.1991.tb12381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Briscoe C. P., Wakelam M. J. The regulation of phospholipase D activity and its role in sn-1,2-diradylglycerol formation in bombesin- and phorbol 12-myristate 13-acetate-stimulated Swiss 3T3 cells. Biochem J. 1991 Dec 1;280(Pt 2):431–438. doi: 10.1042/bj2800431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Palmer S., Plevin R., Wakelam M. J. Mass measurement of inositol 1,4,5-trisphosphate and sn-1,2-diacylglycerol in bombesin-stimulated Swiss 3T3 mouse fibroblasts. Biochem J. 1990 Jan 15;265(2):617–620. doi: 10.1042/bj2650617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Analysis of the water-soluble products of phosphatidylcholine breakdown by ion-exchange chromatography. Bombesin and TPA (12-O-tetradecanoylphorbol 13-acetate) stimulate choline generation in Swiss 3T3 cells by a common mechanism. Biochem J. 1989 Oct 15;263(2):581–587. doi: 10.1042/bj2630581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Hydrolysis of phosphatidylcholine by phospholipase D is a common response to mitogens which stimulate inositol lipid hydrolysis in Swiss 3T3 fibroblasts. Biochim Biophys Acta. 1991 Apr 17;1092(2):265–272. doi: 10.1016/0167-4889(91)90166-u. [DOI] [PubMed] [Google Scholar]
- Davis P. D., Hill C. H., Keech E., Lawton G., Nixon J. S., Sedgwick A. D., Wadsworth J., Westmacott D., Wilkinson S. E. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989 Dec 18;259(1):61–63. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Doyle V. M., Rüegg U. T. Vasopressin induced production of inositol trisphosphate and calcium efflux in a smooth muscle cell line. Biochem Biophys Res Commun. 1985 Aug 30;131(1):469–476. doi: 10.1016/0006-291x(85)91826-1. [DOI] [PubMed] [Google Scholar]
- Griendling K. K., Rittenhouse S. E., Brock T. A., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986 May 5;261(13):5901–5906. [PubMed] [Google Scholar]
- Jard S., Gaillard R. C., Guillon G., Marie J., Schoenenberg P., Muller A. F., Manning M., Sawyer W. H. Vasopressin antagonists allow demonstration of a novel type of vasopressin receptor in the rat adenohypophysis. Mol Pharmacol. 1986 Aug;30(2):171–177. [PubMed] [Google Scholar]
- Lassègue B., Alexander R. W., Clark M., Griendling K. K. Angiotensin II-induced phosphatidylcholine hydrolysis in cultured vascular smooth-muscle cells. Regulation and localization. Biochem J. 1991 May 15;276(Pt 1):19–25. doi: 10.1042/bj2760019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch M., Amsterdam A. Gonadotropin-releasing hormone activates phospholipase D in ovarian granulosa cells. Possible role in signal transduction. J Biol Chem. 1989 Jul 15;264(20):11762–11767. [PubMed] [Google Scholar]
- MacNulty E. E., Plevin R., Wakelam M. J. Stimulation of the hydrolysis of phosphatidylinositol 4,5-bisphosphate and phosphatidylcholine by endothelin, a complete mitogen for Rat-1 fibroblasts. Biochem J. 1990 Dec 15;272(3):761–766. doi: 10.1042/bj2720761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. W., Michaelis K. P2-purinergic agonists stimulate phosphodiesteratic cleavage of phosphatidylcholine in endothelial cells. Evidence for activation of phospholipase D. J Biol Chem. 1989 May 25;264(15):8847–8856. [PubMed] [Google Scholar]
- Martinson E. A., Trilivas I., Brown J. H. Rapid protein kinase C-dependent activation of phospholipase D leads to delayed 1,2-diglyceride accumulation. J Biol Chem. 1990 Dec 25;265(36):22282–22287. [PubMed] [Google Scholar]
- Merritt J. E., Armstrong W. P., Benham C. D., Hallam T. J., Jacob R., Jaxa-Chamiec A., Leigh B. K., McCarthy S. A., Moores K. E., Rink T. J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990 Oct 15;271(2):515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ohanian J., Ollerenshaw J., Collins P., Heagerty A. Agonist-induced production of 1,2-diacylglycerol and phosphatidic acid in intact resistance arteries. Evidence that accumulation of diacylglycerol is not a prerequisite for contraction. J Biol Chem. 1990 May 25;265(15):8921–8928. [PubMed] [Google Scholar]
- Pai J. K., Siegel M. I., Egan R. W., Billah M. M. Phospholipase D catalyzes phospholipid metabolism in chemotactic peptide-stimulated HL-60 granulocytes. J Biol Chem. 1988 Sep 5;263(25):12472–12477. [PubMed] [Google Scholar]
- Palmer S., Hughes K. T., Lee D. Y., Wakelam M. J. Development of a novel, Ins(1,4,5)P3-specific binding assay. Its use to determine the intracellular concentration of Ins(1,4,5)P3 in unstimulated and vasopressin-stimulated rat hepatocytes. Cell Signal. 1989;1(2):147–156. doi: 10.1016/0898-6568(89)90004-1. [DOI] [PubMed] [Google Scholar]
- Penit J., Faure M., Jard S. Vasopressin and angiotensin II receptors in rat aortic smooth muscle cells in culture. Am J Physiol. 1983 Jan;244(1):E72–E82. doi: 10.1152/ajpendo.1983.244.1.E72. [DOI] [PubMed] [Google Scholar]
- Plevin R., Palmer S., Gardner S. D., Wakelam M. J. Regulation of bombesin-stimulated inositol 1,4,5-trisphosphate generation in Swiss 3T3 fibroblasts by a guanine-nucleotide-binding protein. Biochem J. 1990 Jun 15;268(3):605–610. doi: 10.1042/bj2680605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall R. W., Bonser R. W., Thompson N. T., Garland L. G. A novel and sensitive assay for phospholipase D in intact cells. FEBS Lett. 1990 May 7;264(1):87–90. doi: 10.1016/0014-5793(90)80772-b. [DOI] [PubMed] [Google Scholar]
- Rüegg U. T., Wallnöfer A., Weir S., Cauvin C. Receptor-operated calcium-permeable channels in vascular smooth muscle. J Cardiovasc Pharmacol. 1989;14 (Suppl 6):S49–S58. [PubMed] [Google Scholar]
- Sunako M., Kawahara Y., Hirata K., Tsuda T., Yokoyama M., Fukuzaki H., Takai Y. Mass analysis of 1,2-diacylglycerol in cultured rabbit vascular smooth muscle cells. Comparison of stimulation by angiotensin II and endothelin. Hypertension. 1990 Jan;15(1):84–88. doi: 10.1161/01.hyp.15.1.84. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Cook S. J., Currie S., Palmer S., Plevin R. Regulation of the hydrolysis of phosphatidylcholine in Swiss 3T3 cells. Biochem Soc Trans. 1991 Apr;19(2):321–324. doi: 10.1042/bst0190321. [DOI] [PubMed] [Google Scholar]
- Welsh C. J., Schmeichel K., Cao H. T., Chabbott H. Vasopressin stimulates phospholipase D activity against phosphatidylcholine in vascular smooth muscle cells. Lipids. 1990 Nov;25(11):675–684. doi: 10.1007/BF02544033. [DOI] [PubMed] [Google Scholar]
- Zschauer A., Scott-Burden T., Bühler F. R., van Breemen C. Vasopressor peptides and depolarization stimulated Ca2+-entry into cultured vascular smooth muscle. Biochem Biophys Res Commun. 1987 Oct 14;148(1):225–231. doi: 10.1016/0006-291x(87)91099-0. [DOI] [PubMed] [Google Scholar]


