Abstract
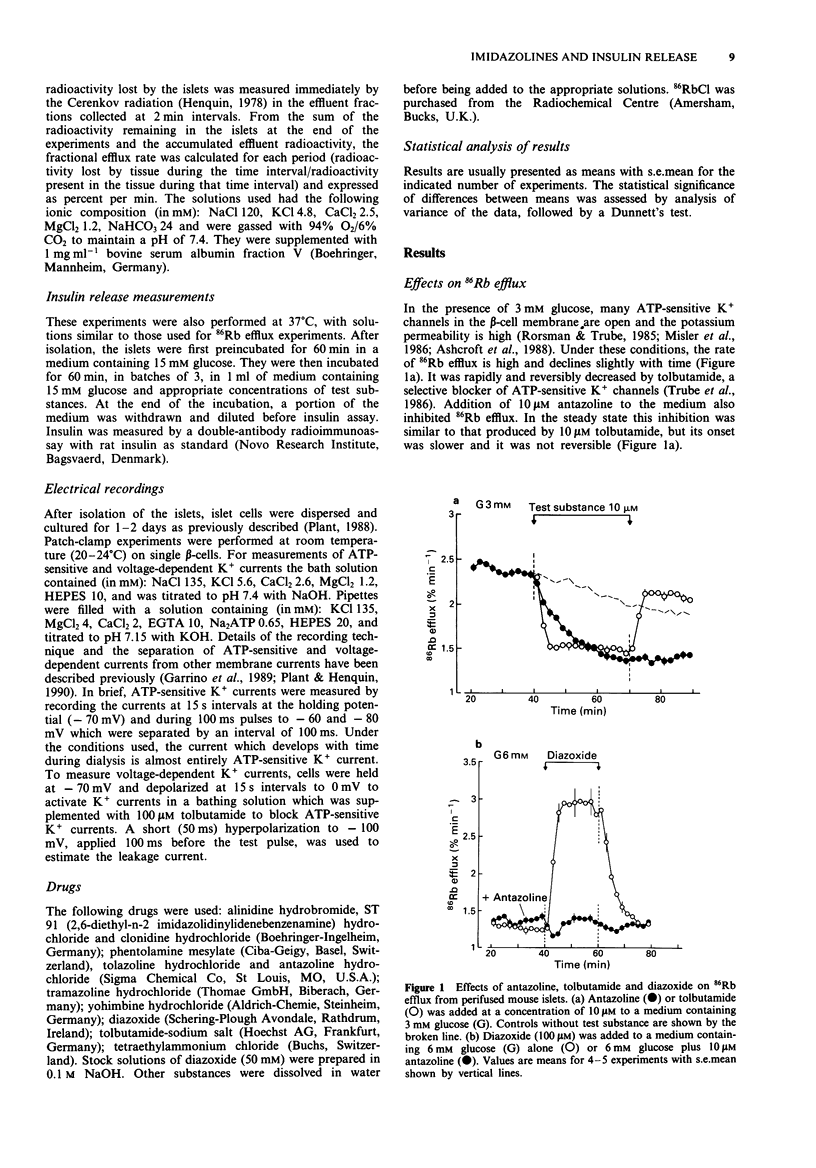
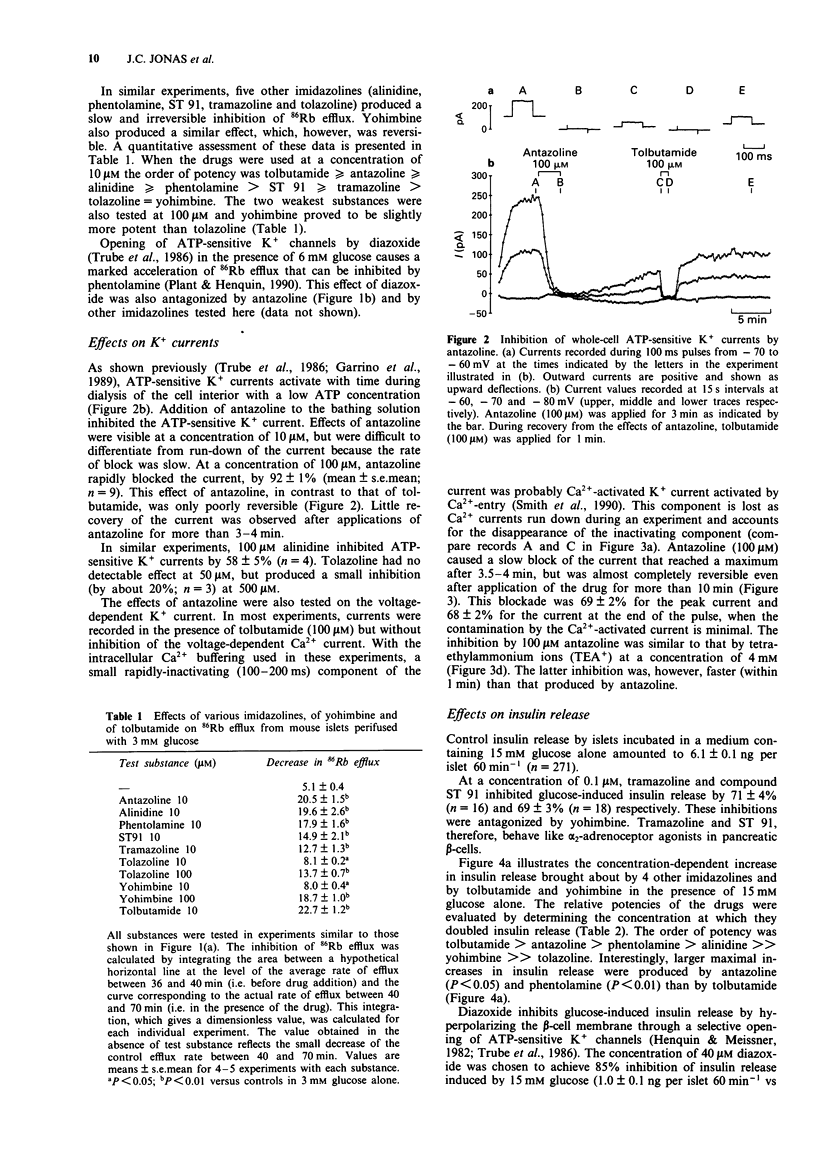
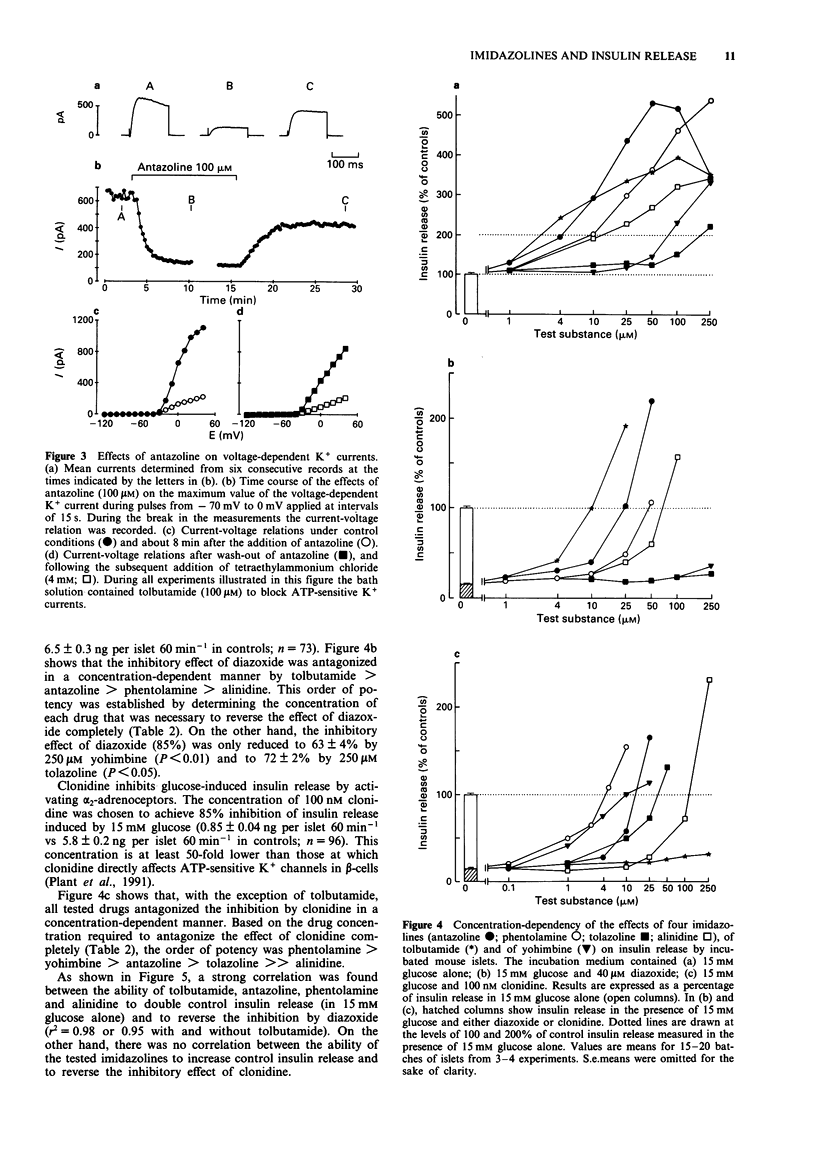
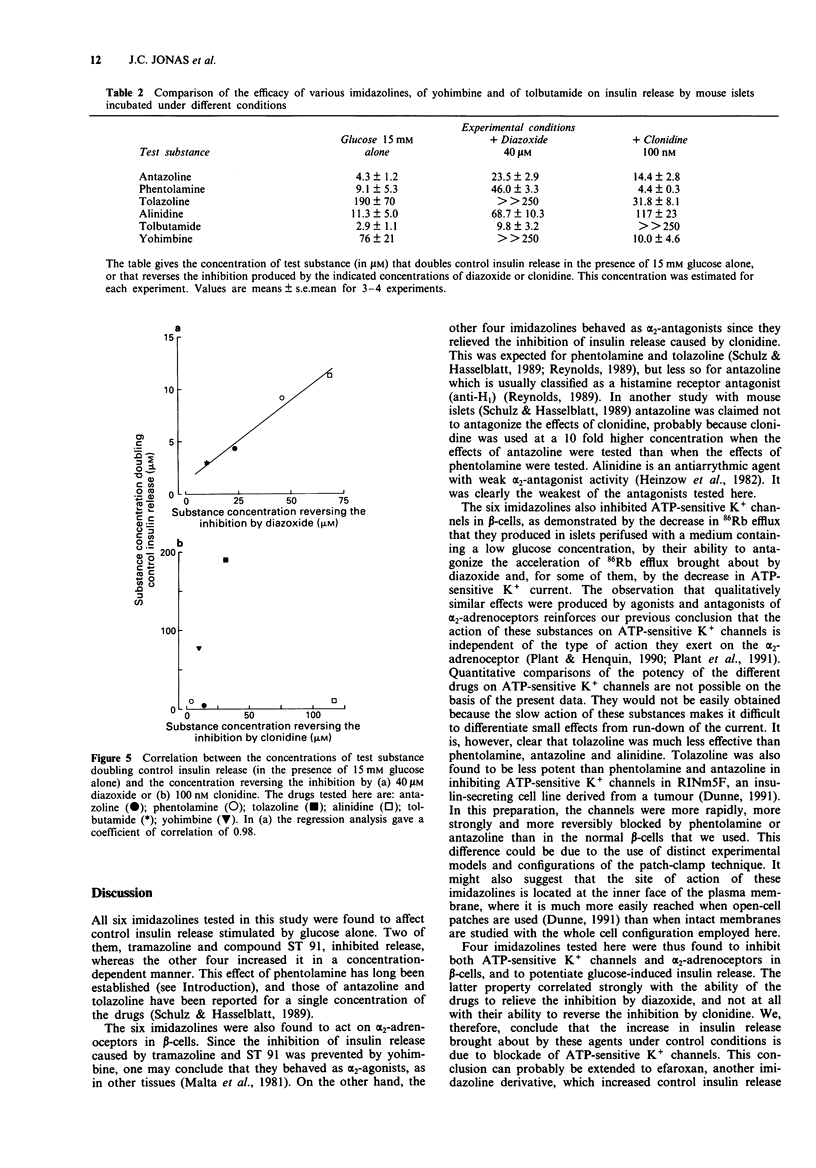
1. Islets from normal mice were used to study the mechanisms by which imidazoline antagonists of alpha 2-adrenoceptors increase insulin release in vitro. 2. Alinidine, antazoline, phentolamine and tolazoline inhibited 86Rb efflux from islets perifused with a medium containing 3 mM glucose, i.e. under conditions where many adenosine 5'-triphosphate (ATP)-sensitive K+ channels are open in the beta-cell membrane. They also reduced the acceleration of 86Rb efflux caused by diazoxide, an opener of ATP-sensitive K+ channels. 3. ATP-sensitive and voltage-sensitive K+ currents were measured in single beta-cells by the whole-cell mode of the patch-clamp technique. Antazoline more markedly inhibited the ATP-sensitive than the voltage-sensitive current, an effect previously observed with phentolamine. Alinidine and tolazoline partially decreased the ATP-sensitive K+ current. 4. The four imidazolines reversed the inhibition of insulin release caused by diazoxide (through opening of ATP-sensitive K+ channels) or by clonidine (through activation of alpha 2-adrenoceptors) in a concentration-dependent manner. Only the former effect correlated with the ability of each drug to increase control insulin release stimulated by 15 mM glucose alone. 5. It is concluded that the ability of imidazoline antagonists of alpha 2-adrenoceptors to increase insulin release in vitro can be ascribed to their blockade of ATP-sensitive K+ channels in beta-cells rather than to their interaction with the adrenoceptor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrén B., Lundquist I. Effects of alpha-adrenoceptor blockade by phentolamine on basal and stimulated insulin secretion in the mouse. Acta Physiol Scand. 1985 Oct;125(2):211–217. doi: 10.1111/j.1748-1716.1985.tb07709.x. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Ashcroft S. J., Harrison D. E. Properties of single potassium channels modulated by glucose in rat pancreatic beta-cells. J Physiol. 1988 Jun;400:501–527. doi: 10.1113/jphysiol.1988.sp017134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas D. Clonidine-displacing substance (CDS) and its putative imidazoline receptor. New leads for further divergence of alpha 2-adrenergic receptor activity. Biochem Pharmacol. 1991 Jun 1;41(11):1541–1549. doi: 10.1016/0006-2952(91)90152-u. [DOI] [PubMed] [Google Scholar]
- Broadstone V. L., Pfeifer M. A., Bajaj V., Stagner J. I., Samols E. Alpha-adrenergic blockade improves glucose-potentiated insulin secretion in non-insulin-dependent diabetes mellitus. Diabetes. 1987 Aug;36(8):932–937. doi: 10.2337/diab.36.8.932. [DOI] [PubMed] [Google Scholar]
- Buse M. G., Johnson A. H., Kuperminc D., Buse J. Effect of alpha-adrenergic blockade on insulin secretion in man. Metabolism. 1970 Mar;19(3):219–225. doi: 10.1016/0026-0495(70)90055-7. [DOI] [PubMed] [Google Scholar]
- Cerasi E., Effendic S., Luft R. Role of adrenergic receptors in glucose-induced insulin secretion in man. Lancet. 1969 Aug 9;2(7615):301–302. doi: 10.1016/s0140-6736(69)90059-2. [DOI] [PubMed] [Google Scholar]
- Chan S. L., Dunne M. J., Stillings M. R., Morgan N. G. The alpha 2-adrenoceptor antagonist efaroxan modulates K+ATP channels in insulin-secreting cells. Eur J Pharmacol. 1991 Oct 29;204(1):41–48. doi: 10.1016/0014-2999(91)90833-c. [DOI] [PubMed] [Google Scholar]
- Chan S. L., Morgan N. G. Stimulation of insulin secretion by efaroxan may involve interaction with potassium channels. Eur J Pharmacol. 1990 Jan 25;176(1):97–101. doi: 10.1016/0014-2999(90)90137-u. [DOI] [PubMed] [Google Scholar]
- Chan S. L., Stillings M. R., Morgan N. G. Mechanisms involved in stimulation of insulin secretion by the hypoglycaemic alpha-adrenergic antagonist, DG-5128. Biochem Biophys Res Commun. 1991 May 15;176(3):1545–1551. doi: 10.1016/0006-291x(91)90463-h. [DOI] [PubMed] [Google Scholar]
- Dunne M. J. Block of ATP-regulated potassium channels by phentolamine and other alpha-adrenoceptor antagonists. Br J Pharmacol. 1991 Aug;103(4):1847–1850. doi: 10.1111/j.1476-5381.1991.tb12340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efendić S., Cerasi E., Luft R. Effect of phentolamine and preperfusion with glucose on insulin release from the isolated perfused pancreas from fasted and fed rats. Diabetologia. 1975 Oct;11(5):407–410. doi: 10.1007/BF00429908. [DOI] [PubMed] [Google Scholar]
- Garrino M. G., Henquin J. C. B cell adrenoceptors and sulphonylurea-induced insulin release in mouse islets. Diabetologia. 1990 Mar;33(3):145–147. doi: 10.1007/BF00404040. [DOI] [PubMed] [Google Scholar]
- Garrino M. G., Henquin J. C. Highly potent and stereoselective effects of the benzoic acid derivative AZ-DF 265 on pancreatic beta-cells. Br J Pharmacol. 1988 Jan;93(1):61–68. doi: 10.1111/j.1476-5381.1988.tb11405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrino M. G., Plant T. D., Henquin J. C. Effects of putative activators of K+ channels in mouse pancreatic beta-cells. Br J Pharmacol. 1989 Nov;98(3):957–965. doi: 10.1111/j.1476-5381.1989.tb14626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzow B. G., Angus J. A., Korner P. I. Effects of alinidine (ST 567) on baroreceptor-heart rate reflexes and its interactions with clonidine on the baroreflex and on the sympathetic terminals of the isolated atrium. Eur J Pharmacol. 1982 Oct 22;84(3-4):177–187. doi: 10.1016/0014-2999(82)90200-x. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. D-glucose inhibits potassium efflux from pancreatic islet cells. Nature. 1978 Jan 19;271(5642):271–273. doi: 10.1038/271271a0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochem Pharmacol. 1982 Apr 1;31(7):1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Kawazu S., Suzuki M., Negishi K., Watanabe T., Ishii J. Studies of midaglizole (DG-5128). A new type of oral hypoglycemic drug in healthy subjects. Diabetes. 1987 Feb;36(2):216–220. doi: 10.2337/diab.36.2.216. [DOI] [PubMed] [Google Scholar]
- Lundquist I. Interaction of amines and aminergic blocking agents with blood glucose regulation. II. Alpha-adrenergic blockade. Eur J Pharmacol. 1972 May;18(2):225–235. doi: 10.1016/0014-2999(72)90246-4. [DOI] [PubMed] [Google Scholar]
- Malta E., Raper C., Tawa P. E. Pre- and postjunctional effects of clonidine- and oxymetazoline-like compounds in guinea-pig ileal preparations. Br J Pharmacol. 1981 Jun;73(2):355–362. doi: 10.1111/j.1476-5381.1981.tb10429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M. C., Insel P. A. Are there multiple imidazoline binding sites? Trends Pharmacol Sci. 1989 Sep;10(9):342–344. doi: 10.1016/0165-6147(89)90002-3. [DOI] [PubMed] [Google Scholar]
- Misbin R. I., Edgar P. J., Lockwood D. H. Adrenergic regulation of insulin secretion during fasting in normal subjects. Diabetes. 1970 Oct;19(10):688–693. doi: 10.2337/diab.19.10.688. [DOI] [PubMed] [Google Scholar]
- Misler S., Falke L. C., Gillis K., McDaniel M. L. A metabolite-regulated potassium channel in rat pancreatic B cells. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7119–7123. doi: 10.1073/pnas.83.18.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaki T., Nakadate T., Kato R. Alpha 2-adrenoceptors modulating insulin release from isolated pancreatic islets. Naunyn Schmiedebergs Arch Pharmacol. 1980 Aug;313(2):151–153. doi: 10.1007/BF00498572. [DOI] [PubMed] [Google Scholar]
- Ortiz-Alonso F. J., Herman W. H., Gertz B. J., Williams V. C., Smith M. J., Halter J. B. Effect of an oral alpha 2-adrenergic blocker (MK-912) on pancreatic islet function in non-insulin-dependent diabetes mellitus. Metabolism. 1991 Nov;40(11):1160–1167. doi: 10.1016/0026-0495(91)90210-n. [DOI] [PubMed] [Google Scholar]
- Plant T. D., Henquin J. C. Phentolamine and yohimbine inhibit ATP-sensitive K+ channels in mouse pancreatic beta-cells. Br J Pharmacol. 1990 Sep;101(1):115–120. doi: 10.1111/j.1476-5381.1990.tb12099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant T. D., Jonas J. C., Henquin J. C. Clonidine inhibits ATP-sensitive K+ channels in mouse pancreatic beta-cells. Br J Pharmacol. 1991 Oct;104(2):385–390. doi: 10.1111/j.1476-5381.1991.tb12440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant T. D. Properties and calcium-dependent inactivation of calcium currents in cultured mouse pancreatic B-cells. J Physiol. 1988 Oct;404:731–747. doi: 10.1113/jphysiol.1988.sp017316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. P., Halter J. B., Porte D., Jr A role for alpha-adrenergic receptors in abnormal insulin secretion in diabetes mellitus. J Clin Invest. 1976 Mar;57(3):791–795. doi: 10.1172/JCI108338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Glucose dependent K+-channels in pancreatic beta-cells are regulated by intracellular ATP. Pflugers Arch. 1985 Dec;405(4):305–309. doi: 10.1007/BF00595682. [DOI] [PubMed] [Google Scholar]
- Schulz A., Hasselblatt A. An insulin-releasing property of imidazoline derivatives is not limited to compounds that block alpha-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1989 Sep;340(3):321–327. doi: 10.1007/BF00168517. [DOI] [PubMed] [Google Scholar]
- Schulz A., Hasselblatt A. Phentolamine, a deceptive tool to investigate sympathetic nervous control of insulin release. Naunyn Schmiedebergs Arch Pharmacol. 1988 Jun;337(6):637–643. doi: 10.1007/BF00175789. [DOI] [PubMed] [Google Scholar]
- Smith M., Furman B. L. Augmentation of glucose induced insulin secretion by pertussis vaccine, phentolamine and benextramine: involvement of mechanisms additional to prevention of the inhibitory actions of catecholamines in rats. Acta Endocrinol (Copenh) 1988 May;118(1):89–95. doi: 10.1530/acta.0.1180089. [DOI] [PubMed] [Google Scholar]
- Smith P. A., Bokvist K., Arkhammar P., Berggren P. O., Rorsman P. Delayed rectifying and calcium-activated K+ channels and their significance for action potential repolarization in mouse pancreatic beta-cells. J Gen Physiol. 1990 Jun;95(6):1041–1059. doi: 10.1085/jgp.95.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]


