Abstract
1. The dependence on extracellular L-arginine of vascular hyporeactivity induced by bacterial lipopolysaccharide (LPS) was studied in vivo in rats infused with LPS and in vitro in endothelium-denuded rat thoracic aortic rings exposed to LPS. 2. Infusion of LPS during 50 min at a dose of 10 mg kg-1 h-1 produced a significant impairment of the pressor effect of noradrenaline, while in tissues collected 60 min after the start of LPS infusion, no significant alteration in either plasma arginine concentration or aortic arginine content was found compared to saline-infused controls (where plasma arginine was 78.5 +/- 7 microM and aortic arginine 394 +/- 124 nmol g-1 tissue). 3. Incubation of isolated, endothelium-denuded aortic rings with LPS (10 micrograms ml-1) in the absence of L-arginine for 4 h at 37 degrees C produced a 6 fold (P < 0.01) rightward shift in the noradrenaline concentration-effect curve compared to polymyxin B (1 micrograms ml-1, a LPS neutralizing agent) and reduced by 15% the maximum observed tension. 4. The presence of L-arginine (100 microM) during the incubation with LPS and throughout the following contraction experiments caused a 15 fold (P < 0.01) increase in the EC50 of noradrenaline and greater depression (45%) of the maximum observed tension compared to polymyxin B-treated controls. Responses in control, non LPS-treated rings were unaffected by the presence of L-arginine. 5. The addition of L-arginine to rings incubated with LPS in the absence of L-arginine and maximally precontracted with noradrenaline (10 microM) induced a dose-dependent relaxation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


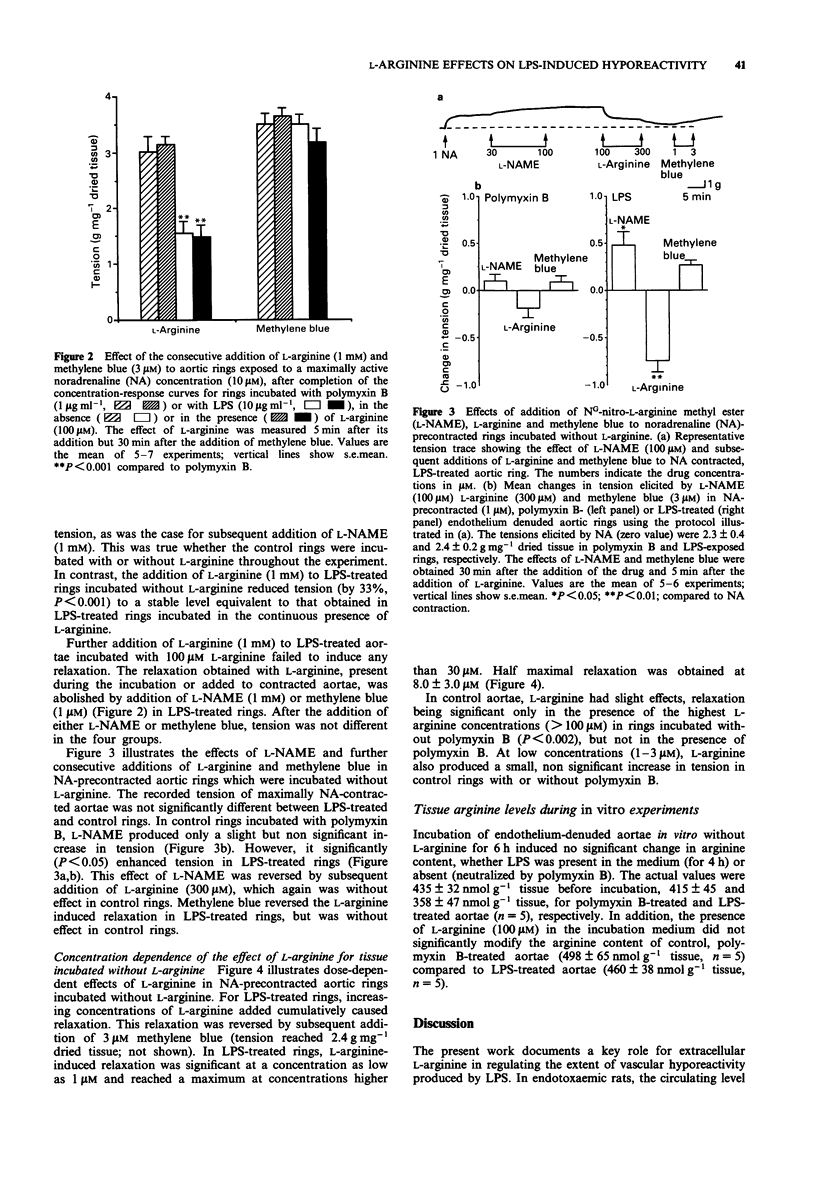
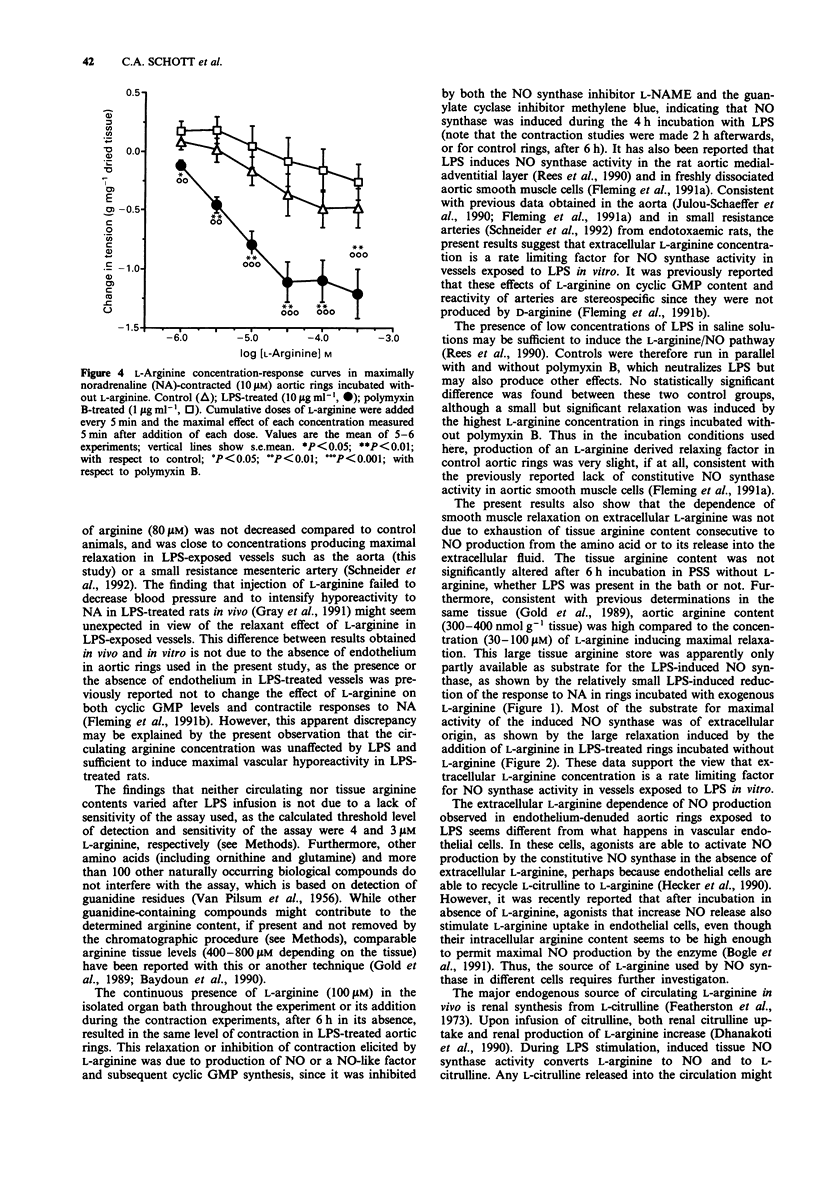

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baydoun A. R., Emery P. W., Pearson J. D., Mann G. E. Substrate-dependent regulation of intracellular amino acid concentrations in cultured bovine aortic endothelial cells. Biochem Biophys Res Commun. 1990 Dec 31;173(3):940–948. doi: 10.1016/s0006-291x(05)80876-9. [DOI] [PubMed] [Google Scholar]
- Bogle R. G., Coade S. B., Moncada S., Pearson J. D., Mann G. E. Bradykinin and ATP stimulate L-arginine uptake and nitric oxide release in vascular endothelial cells. Biochem Biophys Res Commun. 1991 Oct 31;180(2):926–932. doi: 10.1016/s0006-291x(05)81154-4. [DOI] [PubMed] [Google Scholar]
- Dhanakoti S. N., Brosnan J. T., Herzberg G. R., Brosnan M. E. Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol. 1990 Sep;259(3 Pt 1):E437–E442. doi: 10.1152/ajpendo.1990.259.3.E437. [DOI] [PubMed] [Google Scholar]
- Featherston W. R., Rogers Q. R., Freedland R. A. Relative importance of kidney and liver in synthesis of arginine by the rat. Am J Physiol. 1973 Jan;224(1):127–129. doi: 10.1152/ajplegacy.1973.224.1.127. [DOI] [PubMed] [Google Scholar]
- Fleming I., Gray G. A., Julou-Schaeffer G., Parratt J. R., Stoclet J. C. Incubation with endotoxin activates the L-arginine pathway in vascular tissue. Biochem Biophys Res Commun. 1990 Sep 14;171(2):562–568. doi: 10.1016/0006-291x(90)91183-s. [DOI] [PubMed] [Google Scholar]
- Fleming I., Gray G. A., Schott C., Stoclet J. C. Inducible but not constitutive production of nitric oxide by vascular smooth muscle cells. Eur J Pharmacol. 1991 Aug 6;200(2-3):375–376. doi: 10.1016/0014-2999(91)90602-m. [DOI] [PubMed] [Google Scholar]
- Fleming I., Julou-Schaeffer G., Gray G. A., Parratt J. R., Stoclet J. C. Evidence that an L-arginine/nitric oxide dependent elevation of tissue cyclic GMP content is involved in depression of vascular reactivity by endotoxin. Br J Pharmacol. 1991 May;103(1):1047–1052. doi: 10.1111/j.1476-5381.1991.tb12298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn P. M., Shenep J. L., Stokes D. C., Fairclough D., Hildner W. K. Polymyxin B moderates acidosis and hypotension in established, experimental gram-negative septicemia. J Infect Dis. 1987 Nov;156(5):706–712. doi: 10.1093/infdis/156.5.706. [DOI] [PubMed] [Google Scholar]
- Gold M. E., Bush P. A., Ignarro L. J. Depletion of arterial L-arginine causes reversible tolerance to endothelium-dependent relaxation. Biochem Biophys Res Commun. 1989 Oct 31;164(2):714–721. doi: 10.1016/0006-291x(89)91518-0. [DOI] [PubMed] [Google Scholar]
- Gray G. A., Schott C., Julou-Schaeffer G., Fleming I., Parratt J. R., Stoclet J. C. The effect of inhibitors of the L-arginine/nitric oxide pathway on endotoxin-induced loss of vascular responsiveness in anaesthetized rats. Br J Pharmacol. 1991 May;103(1):1218–1224. doi: 10.1111/j.1476-5381.1991.tb12327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld A. B., Bronsveld W., Thijs L. G. Hemodynamic determinants of mortality in human septic shock. Surgery. 1986 Feb;99(2):140–153. [PubMed] [Google Scholar]
- HESS J., KITO E., MARTIN R. P., VAN PILSUM J. F. Determination of creatine, creatinine, arginine, guanidinoacetic acid, guanidine, and methylguanidine in biological fluids. J Biol Chem. 1956 Sep;222(1):225–235. [PubMed] [Google Scholar]
- Hecker M., Sessa W. C., Harris H. J., Anggård E. E., Vane J. R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julou-Schaeffer G., Gray G. A., Fleming I., Schott C., Parratt J. R., Stoclet J. C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Salter M., Brooks S. L., Moncada S. Anti-inflammatory glucocorticoids inhibit the induction by endotoxin of nitric oxide synthase in the lung, liver and aorta of the rat. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1042–1048. doi: 10.1016/0006-291x(90)91551-3. [DOI] [PubMed] [Google Scholar]
- Mann G. E., Pearson J. D., Sheriff C. J., Toothill V. J. Expression of amino acid transport systems in cultured human umbilical vein endothelial cells. J Physiol. 1989 Mar;410:325–339. doi: 10.1113/jphysiol.1989.sp017535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna T. M. Enhanced vascular effects of cyclic GMP in septic rat aorta. Am J Physiol. 1988 Mar;254(3 Pt 2):R436–R442. doi: 10.1152/ajpregu.1988.254.3.R436. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Cellek S., Palmer R. M., Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990 Dec 14;173(2):541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- Schaller M. D., Waeber B., Nussberger J., Brunner H. R. Angiotensin II, vasopressin, and sympathetic activity in conscious rats with endotoxemia. Am J Physiol. 1985 Dec;249(6 Pt 2):H1086–H1092. doi: 10.1152/ajpheart.1985.249.6.H1086. [DOI] [PubMed] [Google Scholar]
- Schini V. B., Vanhoutte P. M. L-arginine evokes both endothelium-dependent and -independent relaxations in L-arginine-depleted aortas of the rat. Circ Res. 1991 Jan;68(1):209–216. doi: 10.1161/01.res.68.1.209. [DOI] [PubMed] [Google Scholar]
- Schneider F., Schott C., Stoclet J. C., Julou-Schaeffer G. L-arginine induces relaxation of small mesenteric arteries from endotoxin-treated rats. Eur J Pharmacol. 1992 Feb 11;211(2):269–272. doi: 10.1016/0014-2999(92)90539-g. [DOI] [PubMed] [Google Scholar]
- Stokes D. C., Shenep J. L., Fishman M., Hildner W. K., Bysani G. K., Rufus K. Polymyxin B prevents lipopolysaccharide-induced release of tumor necrosis factor-alpha from alveolar macrophages. J Infect Dis. 1989 Jul;160(1):52–57. doi: 10.1093/infdis/160.1.52. [DOI] [PubMed] [Google Scholar]
- Wakabayashi I., Hatake K., Kakishita E., Nagai K. Diminution of contractile response of the aorta from endotoxin-injected rats. Eur J Pharmacol. 1987 Sep 2;141(1):117–122. doi: 10.1016/0014-2999(87)90417-1. [DOI] [PubMed] [Google Scholar]


