Abstract
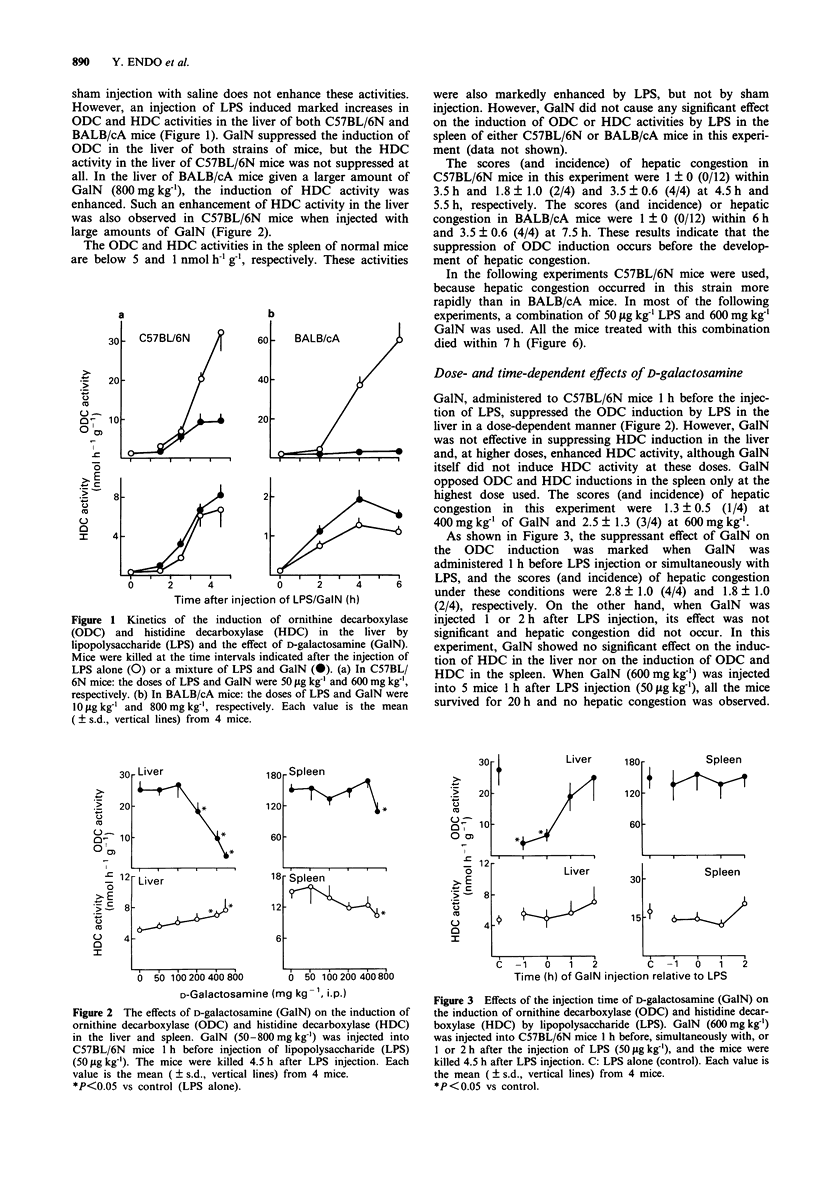
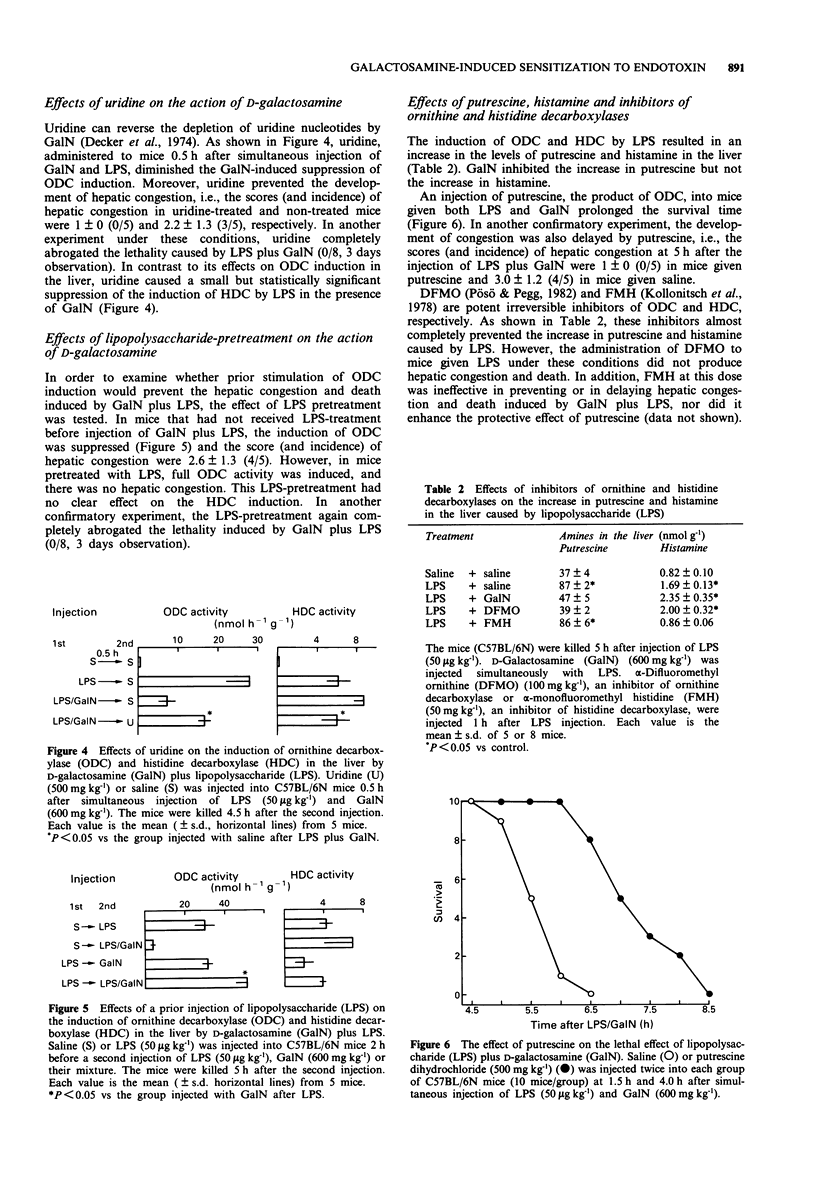
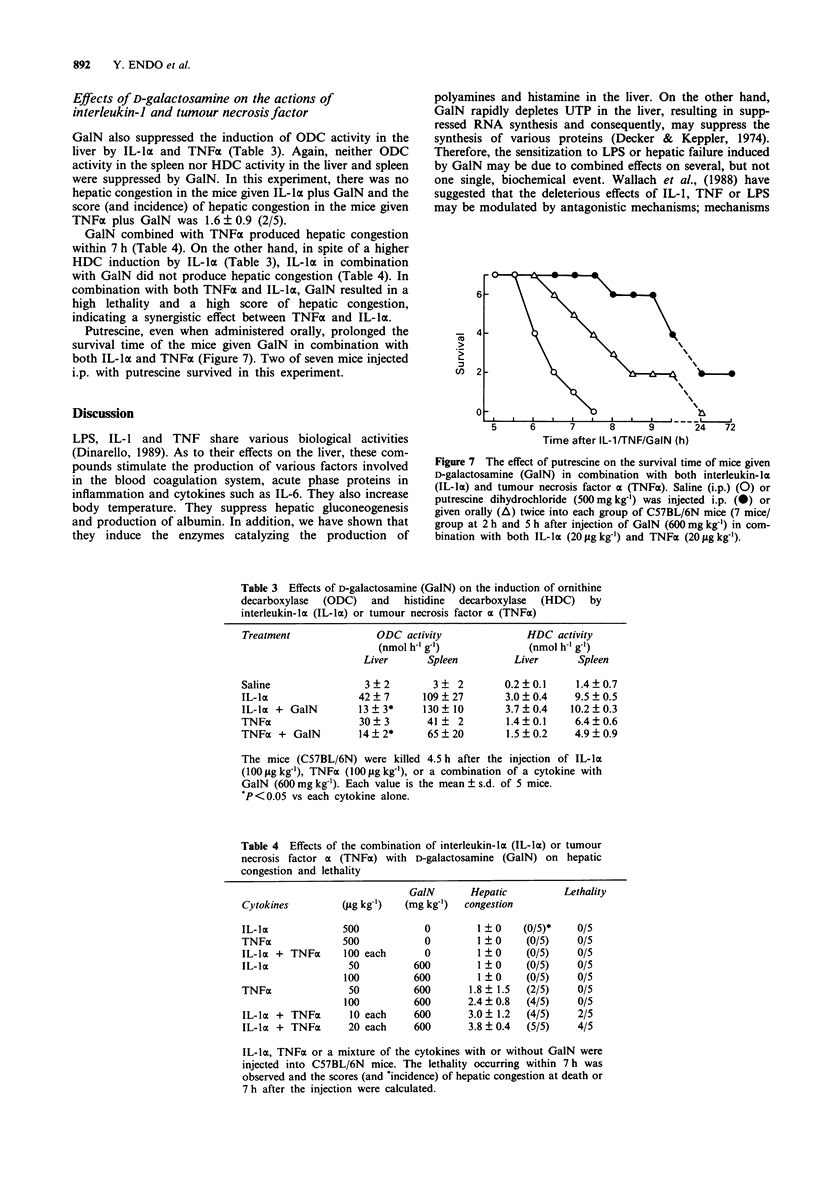
1. An injection of D-galactosamine (GalN) into mice together with a lipopolysaccharide (LPS or endotoxin), interleukin-1 (IL-1) or tumour necrosis factor (TNF), sensitized the mice and induced fulminant hepatitis with severe congestion resulting in rapid death. Since LPS and these cytokines induce ornithine decarboxylase (ODC) and histidine decarboxylase (HDC) in the liver and spleen of mice, the effects of GalN on the induction of ODC and HDC in these organs were examined. 2. The induction of ODC by LPS, IL-1 or TNF was suppressed by GalN in the liver, and this suppression preceded the hepatic congestion. There was good agreement between the degree of hepatic congestion and the suppression of ODC induction by various amounts of GalN. The induction of ODC in the spleen was suppressed only at the highest dose of GalN examined. 3. GalN is known to deplete uridine 5'-triphosphate (UTP), resulting in the suppression of RNA and protein synthesis. An injection of uridine, the precursor of UTP, diminished the GalN-induced suppression of ODC induction by LPS and prevented the hepatic congestion and death. 4. LPS-pretreatment before injection of LPS plus GalN prevented the suppression of ODC activity and prevented the hepatic congestion and death. 5. An injection of putrescine, the product of ODC, prolonged survival time and delayed the development of hepatic congestion. However, injection of an ODC inhibitor into the mice given LPS did not produce hepatic congestion. 6. The induction of HDC in the liver by LPS, IL-1 or TNF was not suppressed by GalN and, at high doses, the response to LPS was enhanced.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Novick D., Hahn T., Fischer D. G., Wallach D. Increase of vulnerability to lymphotoxin in cells infected by vesicular stomatitis virus and its further augmentation by interferon. Cell Immunol. 1985 May;92(2):218–225. doi: 10.1016/0008-8749(85)90003-6. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Daikuhara Y., Tamada F., Takigawa M., Takeda Y., Mori Y. Changes in polyamine metabolism of rat liver after administration of D-galactosamine. Favorable effects of putrescine administration on galactosamine-induced hepatic injury. Gastroenterology. 1979 Jul;77(1):123–132. [PubMed] [Google Scholar]
- Decker K., Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974;(71):77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Endo Y. A simple method for the determination of polyamines and histamine and its application to the assay of ornithine and histidine decarboxylase activities. Methods Enzymol. 1983;94:42–47. doi: 10.1016/s0076-6879(83)94008-9. [DOI] [PubMed] [Google Scholar]
- Endo Y. Induction of histidine and ornithine decarboxylase activities in mouse tissues by recombinant interleukin-1 and tumor necrosis factor. Biochem Pharmacol. 1989 Apr 15;38(8):1287–1292. doi: 10.1016/0006-2952(89)90335-3. [DOI] [PubMed] [Google Scholar]
- Endo Y. Induction of histidine decarboxylase in mouse tissues by mitogens in vivo. Biochem Pharmacol. 1983 Dec 15;32(24):3835–3838. doi: 10.1016/0006-2952(83)90157-0. [DOI] [PubMed] [Google Scholar]
- Endo Y. Induction of ornithine decarboxylase in mouse tissues following the injection of mitogenic substances. Enhancement by actinomycin D. Biochem Pharmacol. 1984 Jul 1;33(13):2123–2127. doi: 10.1016/0006-2952(84)90582-3. [DOI] [PubMed] [Google Scholar]
- Endo Y., Matsushima K., Onozaki K., Oppenheim J. J. Role of ornithine decarboxylase in the regulation of cell growth by IL-1 and tumor necrosis factor. J Immunol. 1988 Oct 1;141(7):2342–2348. [PubMed] [Google Scholar]
- Endo Y., Nakamura M. The effect of lipopolysaccharide, interleukin-1 and tumour necrosis factor on the hepatic accumulation of 5-hydroxytryptamine and platelets in the mouse. Br J Pharmacol. 1992 Mar;105(3):613–619. doi: 10.1111/j.1476-5381.1992.tb09028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y. Simultaneous induction of histidine and ornithine decarboxylases and changes in their product amines following the injection of Escherichia coli lipopolysaccharide into mice. Biochem Pharmacol. 1982 Apr 15;31(8):1643–1647. doi: 10.1016/0006-2952(82)90394-x. [DOI] [PubMed] [Google Scholar]
- Endo Y., Suzuki R., Kumagai K. Induction of ornithine decarboxylase in the liver and spleen of mice by interleukin 1-like factors produced from a macrophage cell line. Biochim Biophys Acta. 1985 Mar 8;838(3):343–350. doi: 10.1016/0304-4165(85)90232-6. [DOI] [PubMed] [Google Scholar]
- Endo Y., Suzuki R., Kumagai K. Macrophages can produce factors capable of inducing histidine decarboxylase, a histamine-forming enzyme, in vivo in the liver, spleen, and lung of mice. Cell Immunol. 1986 Jan;97(1):13–22. doi: 10.1016/0008-8749(86)90370-9. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollonitsch J., Perkins L. M., Patchett A. A., Doldouras G. A., Marburg S., Duggan D. E., Maycock A. L., Aster S. D. Selective inhibitors of biosynthesis of aminergic neurotransmitters. Nature. 1978 Aug 31;274(5674):906–908. doi: 10.1038/274906a0. [DOI] [PubMed] [Google Scholar]
- Lehmann V., Freudenberg M. A., Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med. 1987 Mar 1;165(3):657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk G. D. Essential role of polyamine metabolism in hepatic regeneration. Inhibition of deoxyribonucleic acid and protein synthesis and tissue regeneration by difluoromethylornithine in the rat. Gastroenterology. 1986 May;90(5 Pt 1):1261–1267. doi: 10.1016/0016-5085(86)90394-x. [DOI] [PubMed] [Google Scholar]
- Nishiguchi S., Kuroki T., Takeda T., Nakajima S., Shiomi S., Seki S., Matsui-Yuasa I., Otani S., Kobayashi K. Effects of putrescine on D-galactosamine-induced acute liver failure in rats. Hepatology. 1990 Aug;12(2):348–353. doi: 10.1002/hep.1840120224. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pösö H., Pegg A. E. Effect of alpha-difluoromethylornithine on polyamine and DNA synthesis in regenerating rat liver: reversal of inhibition of DNA synthesis by putrescine. Biochim Biophys Acta. 1982 Feb 26;696(2):179–186. doi: 10.1016/0167-4781(82)90026-4. [DOI] [PubMed] [Google Scholar]
- Tiegs G., Wolter M., Wendel A. Tumor necrosis factor is a terminal mediator in galactosamine/endotoxin-induced hepatitis in mice. Biochem Pharmacol. 1989 Feb 15;38(4):627–631. doi: 10.1016/0006-2952(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Wallach D., Holtmann H., Engelmann H., Nophar Y. Sensitization and desensitization to lethal effects of tumor necrosis factor and IL-1. J Immunol. 1988 May 1;140(9):2994–2999. [PubMed] [Google Scholar]


