Abstract
1. Nonadrenergic, noncholinergic (NANC) nerves mediate vasodilatation in guinea-pig pulmonary artery (PA) by both endothelium-dependent and endothelium-independent mechanisms. The transmitter(s) involved in the endothelium-independent pathway have not yet been identified. We have therefore investigated the possibility that nitric oxide (NO) and guanosine 3',5'-cyclic monophosphate (cyclic GMP) may mediate this neural vasodilator response in guinea-pig branch PA rings denuded of endothelium. 2. Electric field stimulation (EFS, 50 V, 0.2 ms) induced a frequency-dependent (1-24 Hz), tetrodotoxin-sensitive relaxation of the U44069-precontracted PA rings in the presence of adrenergic and cholinergic blockade. 3. The NO synthase inhibitors NG-monomethyl L-arginine (L-NMMA, 100 microM) and NG-nitro L-arginine methyl ester (L-NAME, 30 microM), and the guanylyl cyclase inhibitor methylene blue (5 microM) inhibited the EFS (16 Hz)-induced relaxation by 53 +/- 5, 74 +/- 9 and 82 +/- 9% respectively (n = 5-7, P < 0.01, compared with control rings). 4. Excess concentrations of L-, but not D-arginine (300 microM) completely reversed the inhibitory effect of L-NMMA. 5. The EFS-elicited relaxation (4 Hz) was potentiated by 1 microM zaprinast, a type V phosphodiesterase inhibitor which inhibits guanosine 3':5'-cyclic monophosphate (cyclic GMP) degradation, but was unaffected by 0.1 microM zardaverine, a type III/IV phosphodiesterase inhibitor which inhibits cyclic AMP degradation. 6. EFS (50 V, 0.2 ms, 16 Hz) induced a 3 fold increase in tissue cyclic GMP content, an action which was inhibited by L-NMMA (100 microM).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
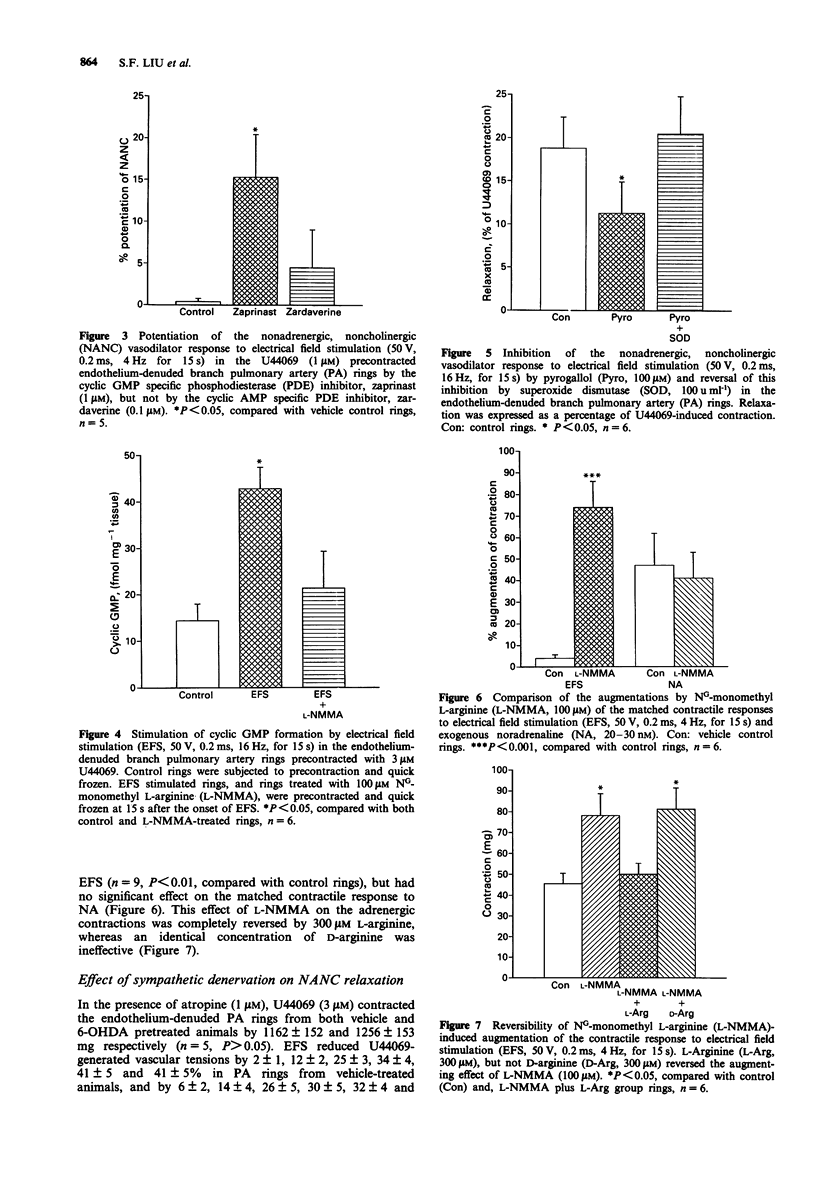
PDF

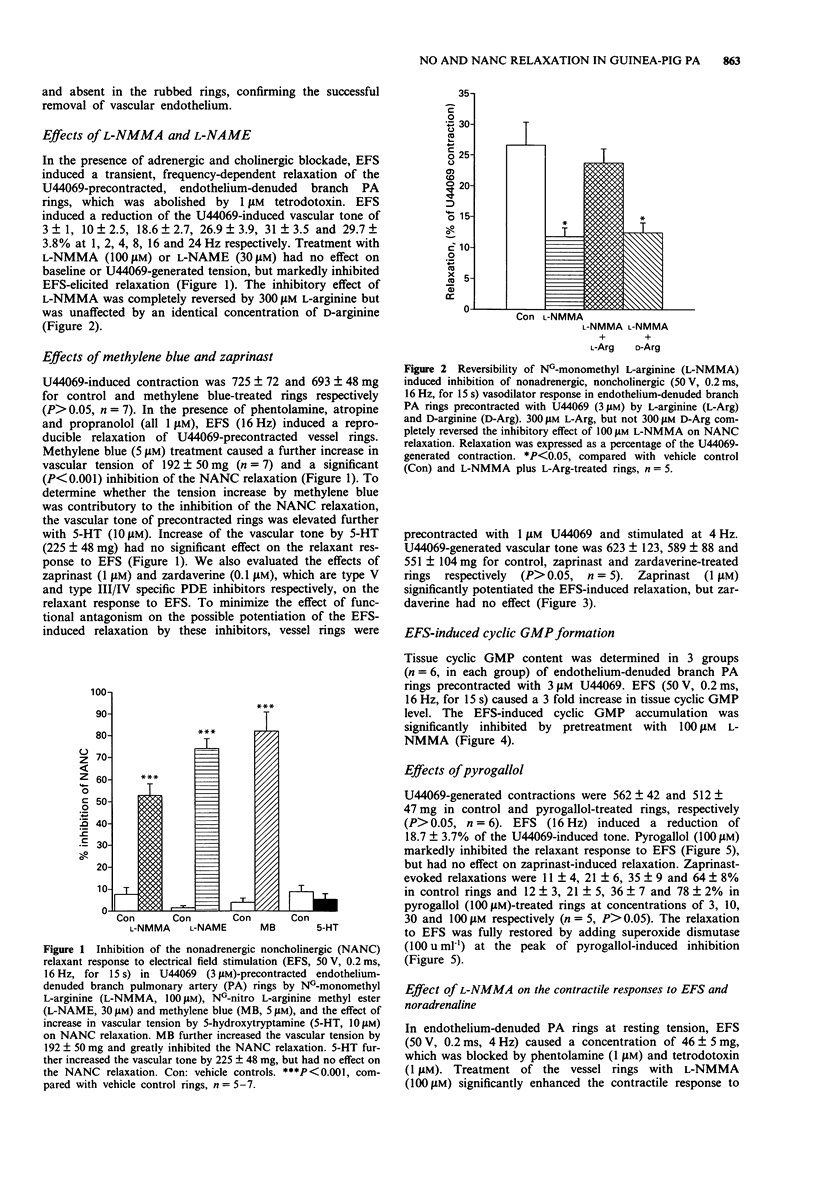


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett T., Burnstock G., Cobb J. L., Malmfors T. An ultrastructural and histochemical study of the short-term effects of 6-hydroxydopamine on adrenergic nerves in the domestic fowl. Br J Pharmacol. 1970 Apr;38(4):802–809. doi: 10.1111/j.1476-5381.1970.tb09889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Clapp L. H. Endothelial-dependent relaxant actions of carbachol and substance P in arterial smooth muscle. Br J Pharmacol. 1986 Apr;87(4):713–723. doi: 10.1111/j.1476-5381.1986.tb14589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Wood K. S. Endothelium-dependent modulation of cGMP levels and intrinsic smooth muscle tone in isolated bovine intrapulmonary artery and vein. Circ Res. 1987 Jan;60(1):82–92. doi: 10.1161/01.res.60.1.82. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989 Jan;3(1):31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- Johns R. A., Peach M. J., Linden J., Tichotsky A. NG-monomethyl L-arginine inhibits endothelium-derived relaxing factor-stimulated cyclic GMP accumulation in cocultures of endothelial and vascular smooth muscle cells by an action specific to the endothelial cell. Circ Res. 1990 Oct;67(4):979–985. doi: 10.1161/01.res.67.4.979. [DOI] [PubMed] [Google Scholar]
- Liu S. F., Crawley D. E., Barnes P. J., Evans T. W. Endothelium-derived relaxing factor inhibits hypoxic pulmonary vasoconstriction in rats. Am Rev Respir Dis. 1991 Jan;143(1):32–37. doi: 10.1164/ajrccm/143.1.32. [DOI] [PubMed] [Google Scholar]
- Liu S. F., Crawley D. E., Evans T. W., Barnes P. J. Endogenous nitric oxide modulates adrenergic neural vasoconstriction in guinea-pig pulmonary artery. Br J Pharmacol. 1991 Oct;104(2):565–569. doi: 10.1111/j.1476-5381.1991.tb12469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. F., Crawley D. E., Evans T. W., Barnes P. J. Endothelium-dependent nonadrenergic, noncholinergic neural relaxation in guinea pig pulmonary artery. J Pharmacol Exp Ther. 1992 Feb;260(2):541–548. [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Perretti F., Tramontana M., Manzini S., Geppetti P., Santicioli P. Sensory nerves, vascular endothelium and neurogenic relaxation of the guinea-pig isolated pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jul;342(1):78–84. doi: 10.1007/BF00178976. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Gryglewski R. J. Mechanism of action of some inhibitors of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9164–9168. doi: 10.1073/pnas.83.23.9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C. D., Challiss R. A., Shahid M. Differential modulation of tissue function and therapeutic potential of selective inhibitors of cyclic nucleotide phosphodiesterase isoenzymes. Trends Pharmacol Sci. 1991 Jan;12(1):19–27. doi: 10.1016/0165-6147(91)90484-a. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Rand M. J. Nitrergic transmission: nitric oxide as a mediator of non-adrenergic, non-cholinergic neuro-effector transmission. Clin Exp Pharmacol Physiol. 1992 Mar;19(3):147–169. doi: 10.1111/j.1440-1681.1992.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Nau H., Wittfoht W., Gerlach J., Prescher K. E., Klein M. M., Niroomand F., Böhme E. Arginine is a physiological precursor of endothelium-derived nitric oxide. Eur J Pharmacol. 1988 Sep 13;154(2):213–216. doi: 10.1016/0014-2999(88)90101-x. [DOI] [PubMed] [Google Scholar]
- Tranzer J. P., Thoenen H. An electron microscopic study of selective, acute degeneration of sympathetic nerve terminals after administration of 6-hydroxydopamine. Experientia. 1968 Feb 15;24(2):155–156. doi: 10.1007/BF02146956. [DOI] [PubMed] [Google Scholar]
- Wood K. S., Buga G. M., Byrns R. E., Ignarro L. J. Vascular smooth muscle-derived relaxing factor (MDRF) and its close similarity to nitric oxide. Biochem Biophys Res Commun. 1990 Jul 16;170(1):80–88. doi: 10.1016/0006-291x(90)91243-l. [DOI] [PubMed] [Google Scholar]


