Abstract
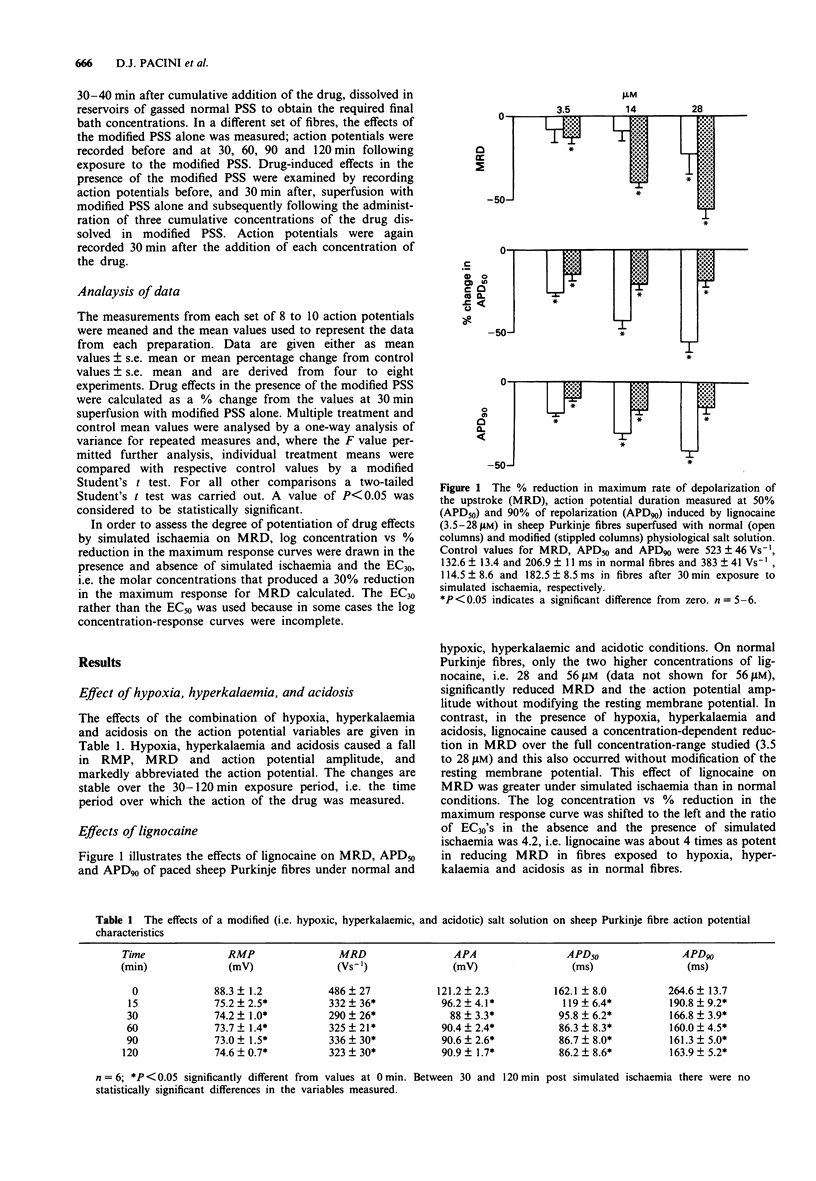
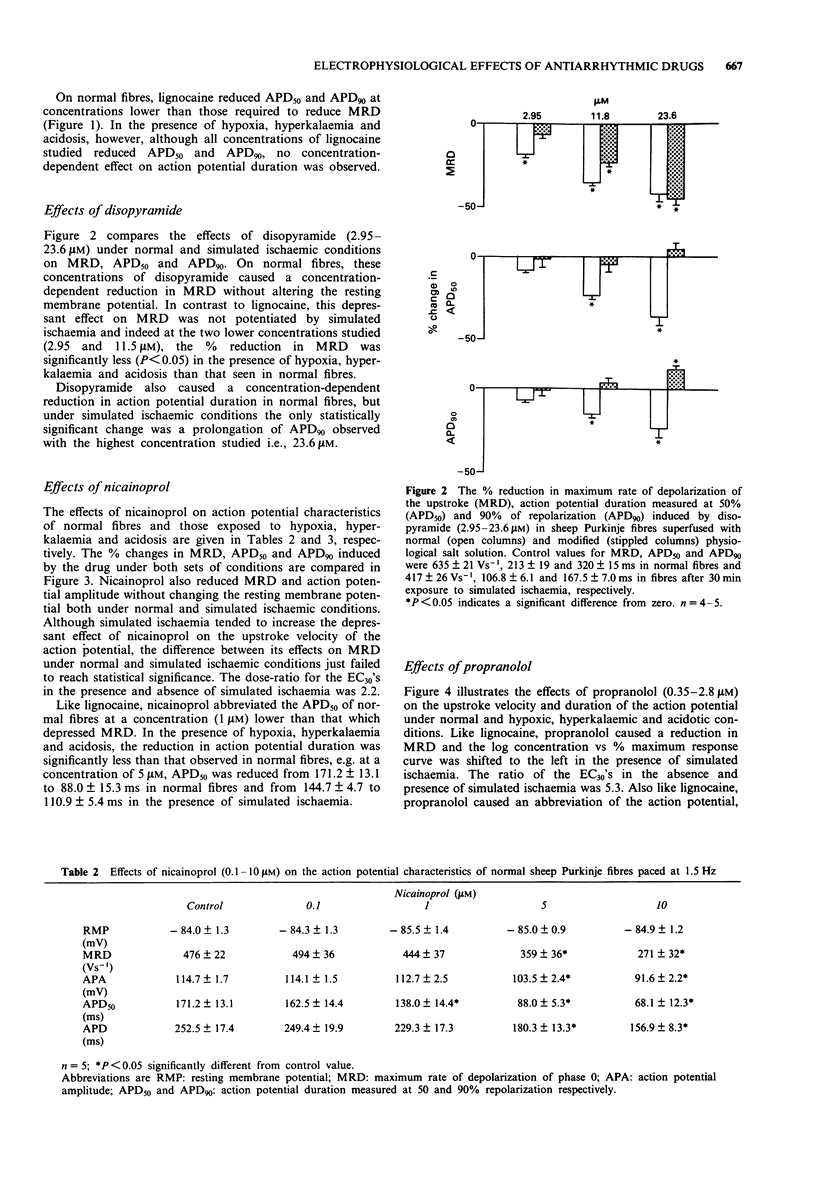
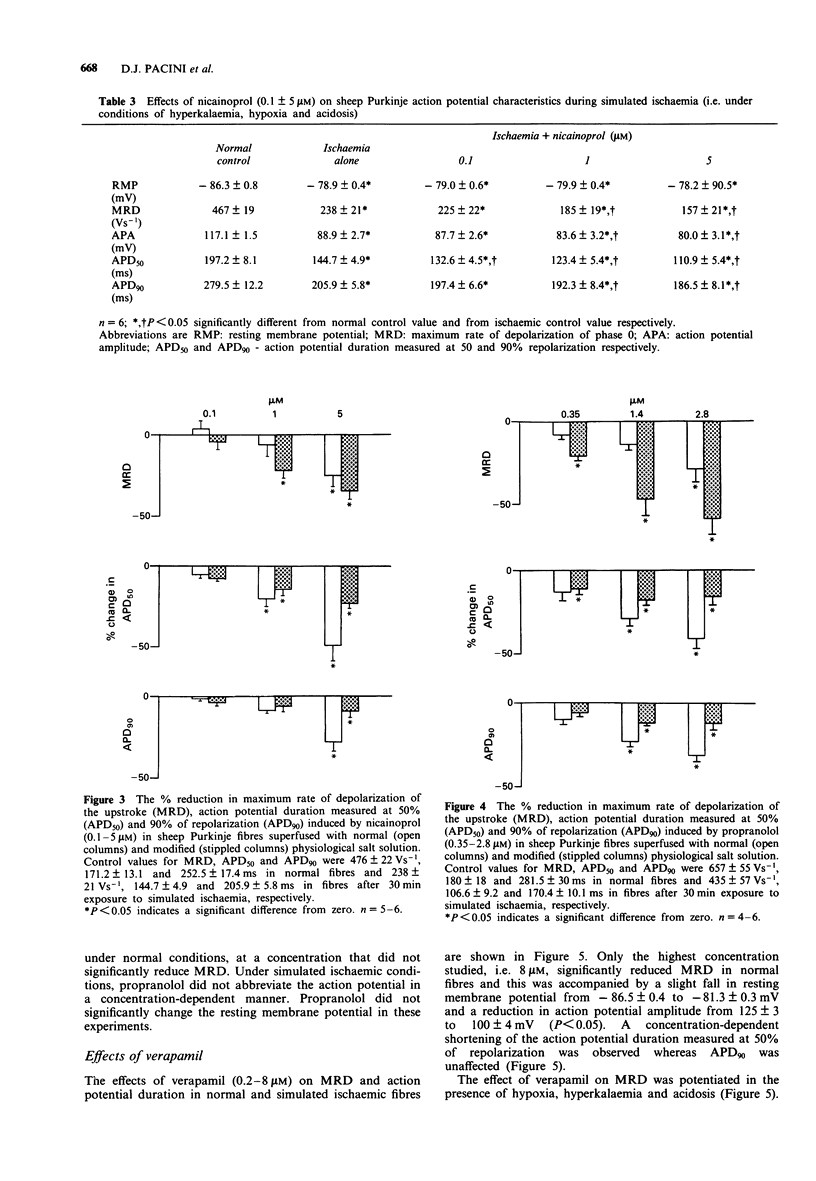
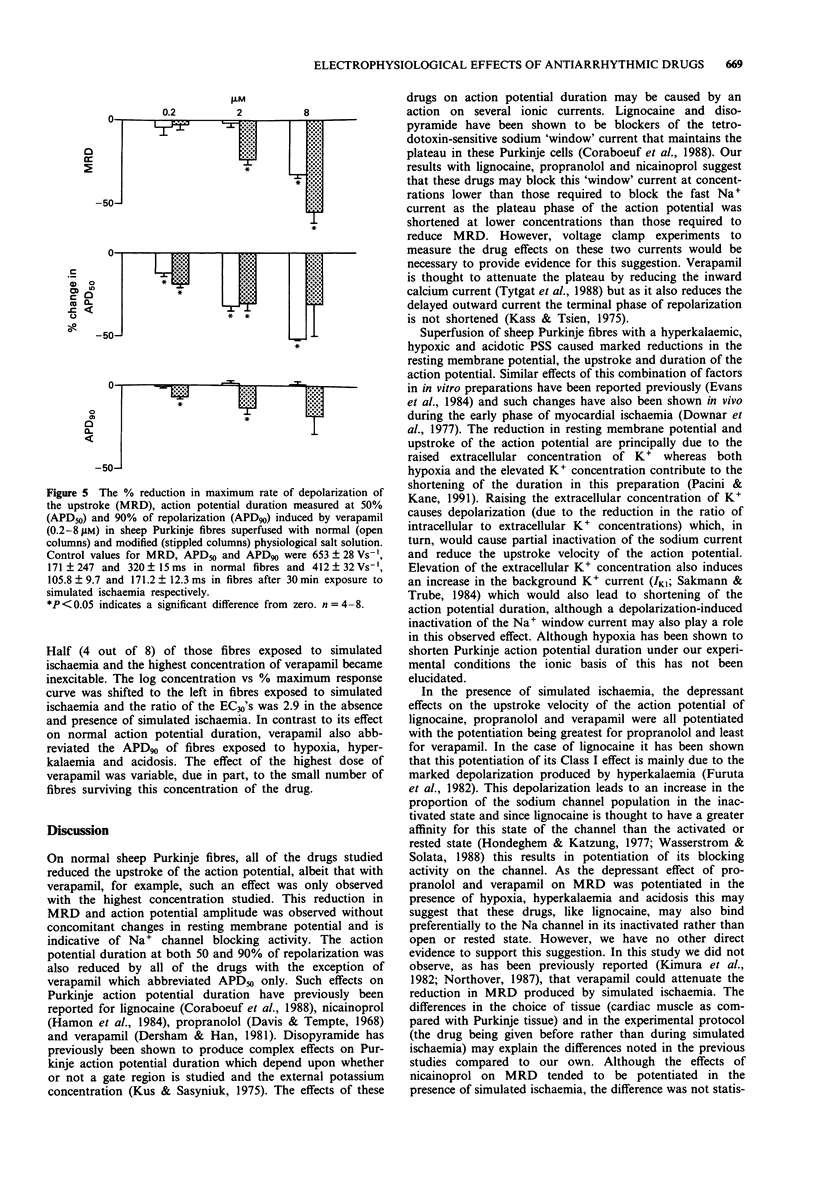
1. The electrophysiological effects of a series of drugs with Class I antiarrhythmic activity were examined in sheep Purkinje fibres, superfused in vitro with either a normal or hypoxic, hyperkalaemic and acidotic physiological salt solution (PSS). 2. In normal sheep Purkinje fibres, lignocaine, disopyramide, nicainoprol and propranolol all significantly reduced action potential height and the maximum rate of depolarization of phase zero (MRD) and abbreviated the action potential, without modifying resting membrane potential (RMP). 3. Verapamil at the highest concentration studied, 8 microM, significantly reduced MRD with an associated slight membrane depolarization and abbreviated action potential duration measured at 50% repolarization (APD50). 4. Superfusion of sheep Purkinje fibres with a hypoxic, hyperkalaemic and acidotic PSS resulted in marked reductions in resting membrane potential, upstroke and duration of the action potential. 5. In the presence of modified PSS, lignocaine, propranolol and verapamil all reduced MRD to a greater extent than in normal PSS. The effects of nicainoprol on MRD were not affected whereas those of disopyramide were significantly attenuated. 6. Under simulated ischaemic conditions, lignocaine, propranolol and nicainoprol did not produce a concentration-dependent reduction in action potential duration whereas disopyramide and verapamil, respectively, prolonged and abbreviated both APD50 and APD90. 7. The Na+ channel blocking actions of the different subtypes of Class I antiarrhythmic agents studied, as well as their effects on action potential duration, were modified differently by simulated ischaemia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P., Cohen C. J., Tsien R. W. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983 May;81(5):613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell T. J. Kinetics of onset of rate-dependent effects of Class I antiarrhythmic drugs are important in determining their effects on refractoriness in guinea-pig ventricle, and provide a theoretical basis for their subclassification. Cardiovasc Res. 1983 Jun;17(6):344–352. doi: 10.1093/cvr/17.6.344. [DOI] [PubMed] [Google Scholar]
- Campbell T. J. Resting and rate-dependent depression of maximum rate of depolarisation (Vmax) in guinea pig ventricular action potentials by mexiletine, disopyramide, and encainide. J Cardiovasc Pharmacol. 1983 Mar-Apr;5(2):291–296. doi: 10.1097/00005344-198303000-00021. [DOI] [PubMed] [Google Scholar]
- Coraboeuf E., Deroubaix E., Escande D., Coulombe A. Comparative effects of three class I antiarrhythmic drugs on plateau and pacemaker currents of sheep cardiac Purkinje fibres. Cardiovasc Res. 1988 Jun;22(6):375–384. doi: 10.1093/cvr/22.6.375. [DOI] [PubMed] [Google Scholar]
- Davis L. D., Temte J. V. Effects of propranolol on the transmembrane potentials of ventricular muscle and Purkinje fibers of the dog. Circ Res. 1968 May;22(5):661–677. doi: 10.1161/01.res.22.5.661. [DOI] [PubMed] [Google Scholar]
- Dersham G. H., Han J. Actions of verapamil on Purkinje fibers from normal and infarcted heart tissues. J Pharmacol Exp Ther. 1981 Feb;216(2):261–264. [PubMed] [Google Scholar]
- Downar E., Janse M. J., Durrer D. The effect of acute coronary artery occlusion on subepicardial transmembrane potentials in the intact porcine heart. Circulation. 1977 Aug;56(2):217–224. doi: 10.1161/01.cir.56.2.217. [DOI] [PubMed] [Google Scholar]
- Evans J. J., Gilmour R. F., Jr, Zipes D. P. The effects of lidocaine and quinidine on impulse propagation across the canine Purkinje-muscle junction during combined hyperkalemia, hypoxia, and acidosis. Circ Res. 1984 Aug;55(2):185–196. doi: 10.1161/01.res.55.2.185. [DOI] [PubMed] [Google Scholar]
- Gruber R., Carmeliet E. The activation gate of the sodium channel controls blockade and deblockade by disopyramide in rabbit Purkinje fibres. Br J Pharmacol. 1989 May;97(1):41–50. doi: 10.1111/j.1476-5381.1989.tb11921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. C. Antiarrhythmic drug classification: new science and practical applications. Am J Cardiol. 1985 Jul 1;56(1):185–187. doi: 10.1016/0002-9149(85)90591-0. [DOI] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977 Nov 14;472(3-4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Multiple effects of calcium antagonists on plateau currents in cardiac Purkinje fibers. J Gen Physiol. 1975 Aug;66(2):169–192. doi: 10.1085/jgp.66.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Nakaya H., Kanno M. Effects of verapamil and lidocaine on changes in action potential characteristics and conduction time induced by combined hypoxia, hyperkalemia, and acidosis in canine ventricular myocardium. J Cardiovasc Pharmacol. 1982 Jul-Aug;4(4):658–667. doi: 10.1097/00005344-198207000-00019. [DOI] [PubMed] [Google Scholar]
- Kimura T., Imanishi S., Arita M. Electrophysiologic effects of nicainoprol, a putative class I antiarrhythmic agent, on the guinea pig ventricular papillary muscle. J Cardiovasc Pharmacol. 1989 May;13(5):767–773. [PubMed] [Google Scholar]
- Kus T., Sasyniuk B. I. The electrophysiological effects of disopyramide phosphate on canine ventricular muscle and Purkinje fibers in normal and low potassium. Can J Physiol Pharmacol. 1978 Feb;56(1):139–149. doi: 10.1139/y78-018. [DOI] [PubMed] [Google Scholar]
- Northover B. J. Electrical changes produced by injury to the rat myocardium in vitro and the protective effects of certain antiarrhythmic drugs. Br J Pharmacol. 1987 Jan;90(1):131–138. doi: 10.1111/j.1476-5381.1987.tb16832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini D. J., Kane K. A. Effects of components of myocardial ischaemia on cardiac action potentials in vitro. J Cardiovasc Pharmacol. 1991 Aug;18(2):261–266. doi: 10.1097/00005344-199108000-00013. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat J., Vereecke J., Carmeliet E. Differential effects of verapamil and flunarizine on cardiac L-type and T-type Ca channels. Naunyn Schmiedebergs Arch Pharmacol. 1988 Jun;337(6):690–692. doi: 10.1007/BF00175798. [DOI] [PubMed] [Google Scholar]
- Weirich J., Antoni H. Evaluation and interpretation of voltage- and frequency-dependent electrophysiologic effects of a new class I antiarrhythmic agent (nicainoprol) on guinea pig papillary muscle and isolated heart. J Cardiovasc Pharmacol. 1988 Dec;12(6):664–671. doi: 10.1097/00005344-198812000-00007. [DOI] [PubMed] [Google Scholar]


