Abstract
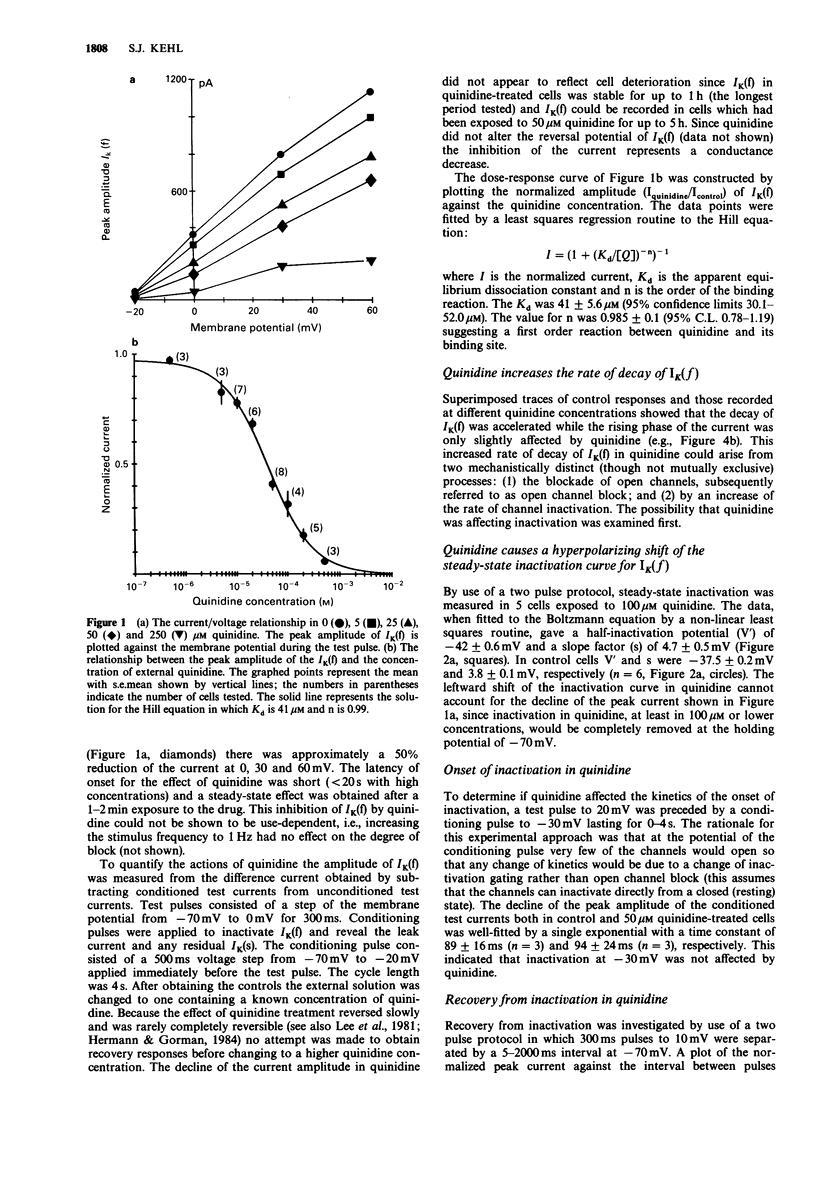
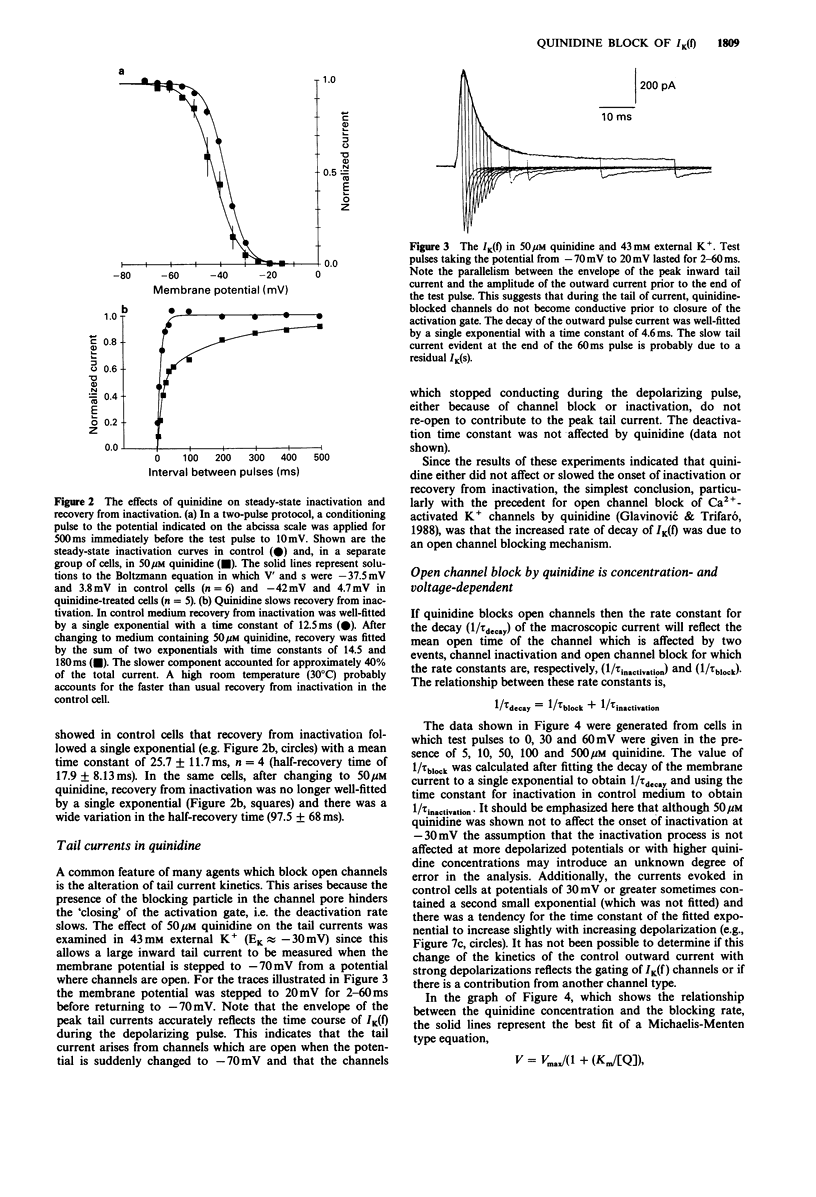
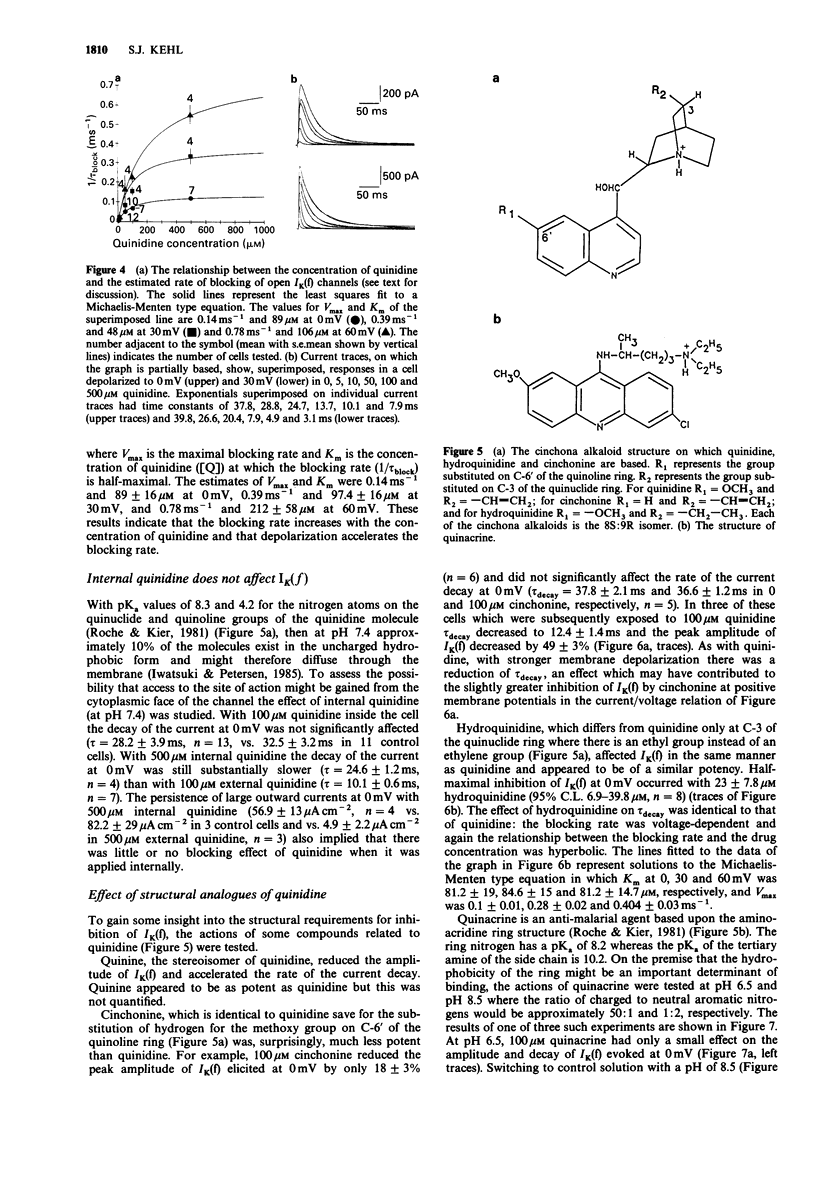
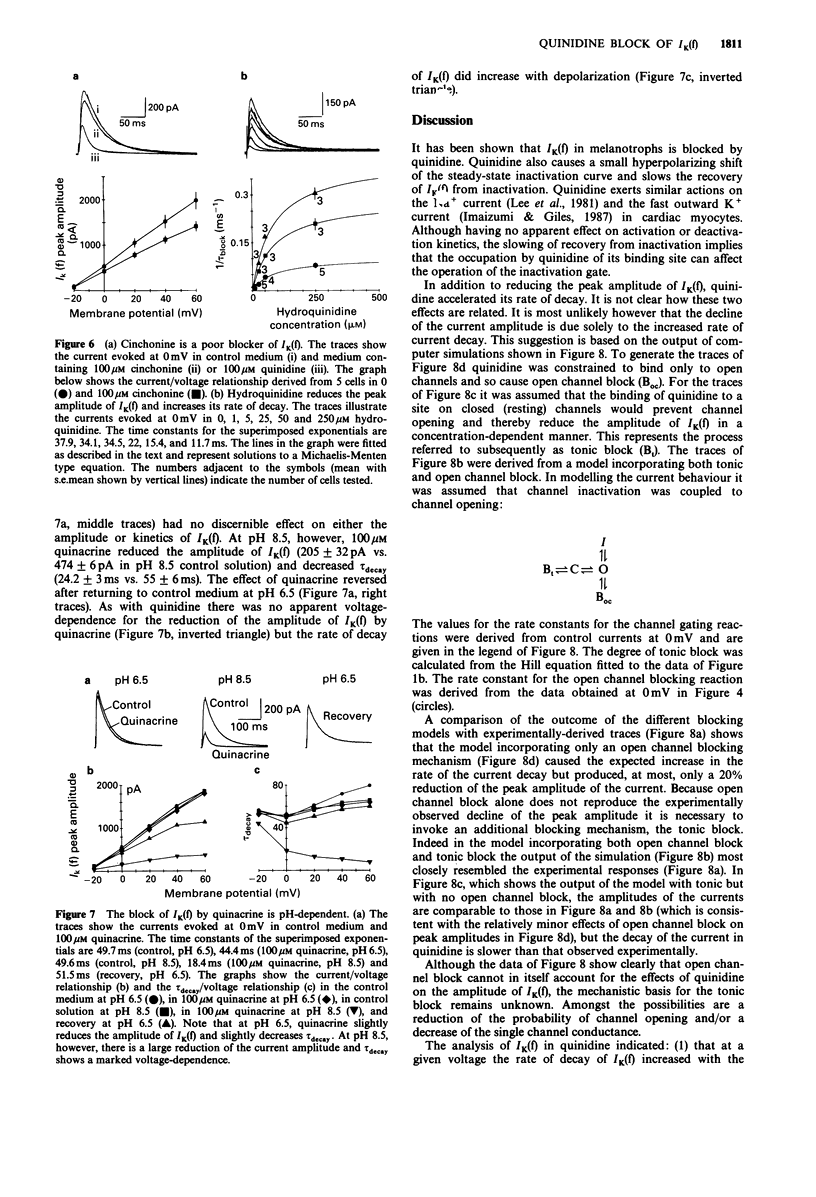
1. The effect of quinidine on the fast-activating, fast-inactivating potassium current (IK(f] in acutely dissociated melanotrophs of the adult rat pituitary was examined. Macroscopic currents were measured by use of the whole-cell configuration of the patch clamp technique. 2. Bath application of quinidine caused a dose-dependent reduction of the peak amplitude of IK(f). The Kd for blockade of IK(f) at 0 mV was estimated to be 41 +/- 5.6 microM. 3. Quinidine elicited a dose-dependent increase of the rate of the decay of IK(f) and this effect was enhanced by membrane depolarization. The possibility that this phenomenon reflects an open channel blocking reaction is discussed. 4. Quinidine also caused a 5 mV hyperpolarizing shift of the steady-state inactivation curve and increased the half-time for recovery from inactivation. Quinidine did not affect the onset of inactivation measured at -30 mV. 5. Internal quinidine did not appear substantially to affect either the peak amplitude or kinetics of IK(f). 6. A study of some structural analogues showed that hydroquinidine and quinacrine had effects similar to those of quinidine. The effect of quinacrine on the amplitude and kinetics of IK(f) was also pH-dependent. Cinchonine, which bears a close structural resemblance to quinidine, was much less effective as a blocker of IK(f).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D., Almers W. Interactions between quaternary lidocaine, the sodium channel gates, and tetrodotoxin. Biophys J. 1979 Jul;27(1):39–55. doi: 10.1016/S0006-3495(79)85201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes I. D., Morad M. Tedisamil inactivates transient outward K+ current in rat ventricular myocytes. Am J Physiol. 1989 Nov;257(5 Pt 2):H1746–H1749. doi: 10.1152/ajpheart.1989.257.5.H1746. [DOI] [PubMed] [Google Scholar]
- Fishman M. C., Spector I. Potassium current suppression by quinidine reveals additional calcium currents in neuroblastoma cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5245–5249. doi: 10.1073/pnas.78.8.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Action of quinidine on ionic currents of molluscan pacemaker neurons. J Gen Physiol. 1984 Jun;83(6):919–940. doi: 10.1085/jgp.83.6.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Giles W. R. Quinidine-induced inhibition of transient outward current in cardiac muscle. Am J Physiol. 1987 Sep;253(3 Pt 2):H704–H708. doi: 10.1152/ajpheart.1987.253.3.H704. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Inhibition of Ca2+-activated K+ channels in pig pancreatic acinar cells by Ba2+, Ca2+, quinine and quinidine. Biochim Biophys Acta. 1985 Oct 10;819(2):249–257. doi: 10.1016/0005-2736(85)90180-4. [DOI] [PubMed] [Google Scholar]
- Kehl S. J. 4-Aminopyridine causes a voltage-dependent block of the transient outward K+ current in rat melanotrophs. J Physiol. 1990 Dec;431:515–528. doi: 10.1113/jphysiol.1990.sp018344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl S. J. Cultured melanotrophs of the adult rat pituitary possess a voltage-activated fast transient outward current. J Physiol. 1989 Apr;411:457–468. doi: 10.1113/jphysiol.1989.sp017583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Hume J. R., Giles W., Brown A. M. Sodium current depression by lidocaine and quinidine in isolated ventricular cells. Nature. 1981 May 28;291(5813):325–327. doi: 10.1038/291325a0. [DOI] [PubMed] [Google Scholar]
- McBurney R. N., Kehl S. J. Electrophysiology of neurosecretory cells from the pituitary intermediate lobe. J Exp Biol. 1988 Sep;139:317–328. doi: 10.1242/jeb.139.1.317. [DOI] [PubMed] [Google Scholar]
- Reichstein E., Rothstein A. Effects of quinine on Ca++-induced K+ efflux from human red blood cells. J Membr Biol. 1981 Mar 15;59(1):57–63. doi: 10.1007/BF01870821. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]


