Abstract
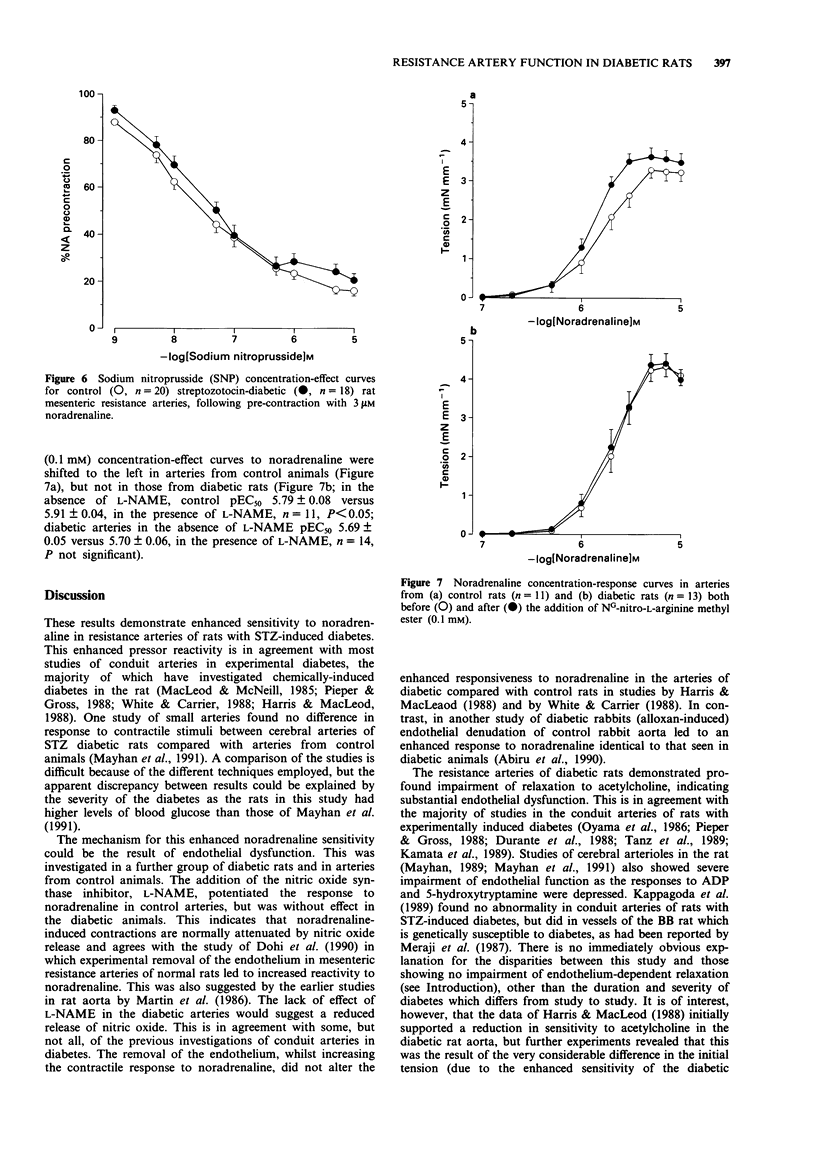
1. Noradrenaline sensitivity and acetylcholine-induced relaxation were investigated in mesenteric resistance arteries of control and streptozotocin-induced diabetic rats. 2. The diabetic rats demonstrated enhanced vascular sensitivity to noradrenaline compared with age-matched controls (pEC50 5.99 +/- 0.06 for diabetic rats, n = 25, versus 5.82 +/- 0.03 for controls, n = 45, P < 0.05). 3. Significant impairment of acetylcholine-induced relaxation was observed in arteries from the diabetic animals compared with controls (pEC50 6.81 +/- 0.17 for diabetic rats, n = 21, versus 7.54 +/- 0.17 for controls, n = 45, P < 0.001). 4. The difference between acetylcholine-induced relaxation in diabetic and control arteries remained in the presence of 10 microM indomethacin (pEC50 6.41 +/- 0.11 for diabetic rats, n = 16, versus 7.59 +/- 0.08 for controls, n = 20, P < 0.001). 5. The nitric oxide synthase inhibitor, NG-monomethyl-L-arginine (L-NMMA, 1 mM) produced profound inhibition of acetylcholine-induced relaxation in diabetic arteries but partial inhibition in controls. The incomplete inhibition of acetylcholine-induced relaxation by L-NMMA in the control arteries was the result of ineffective inhibition of nitric oxide synthase since an alternative inhibitor, NG-nitro-L-arginine methyl ester (L-NAME, 0.1 mM), led to similar inhibition to that seen in the diabetic arteries with L-NMMA. The endothelium-derived relaxing factor (EDRF)-mediated component of acetylcholine-induced relaxation determined by use of the nitric oxide synthase inhibitors was, therefore, apparently reduced in diabetic rats compared with control animals.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

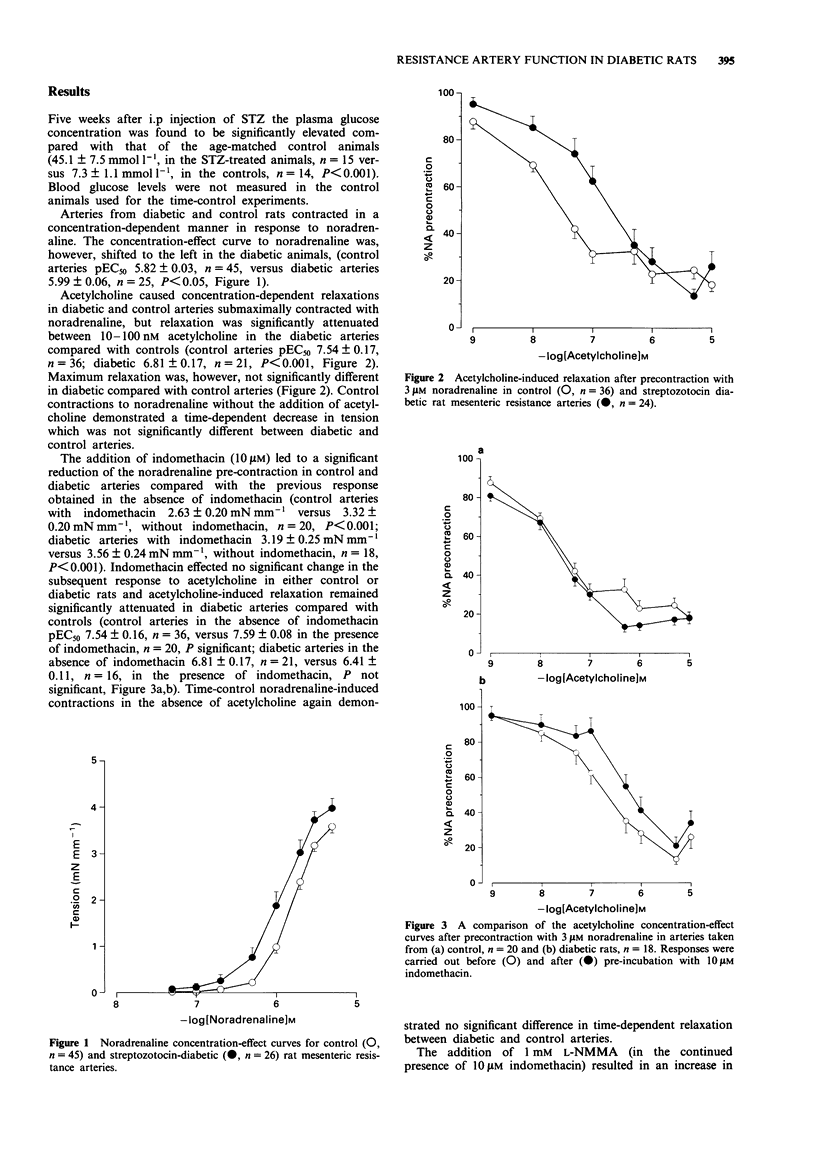
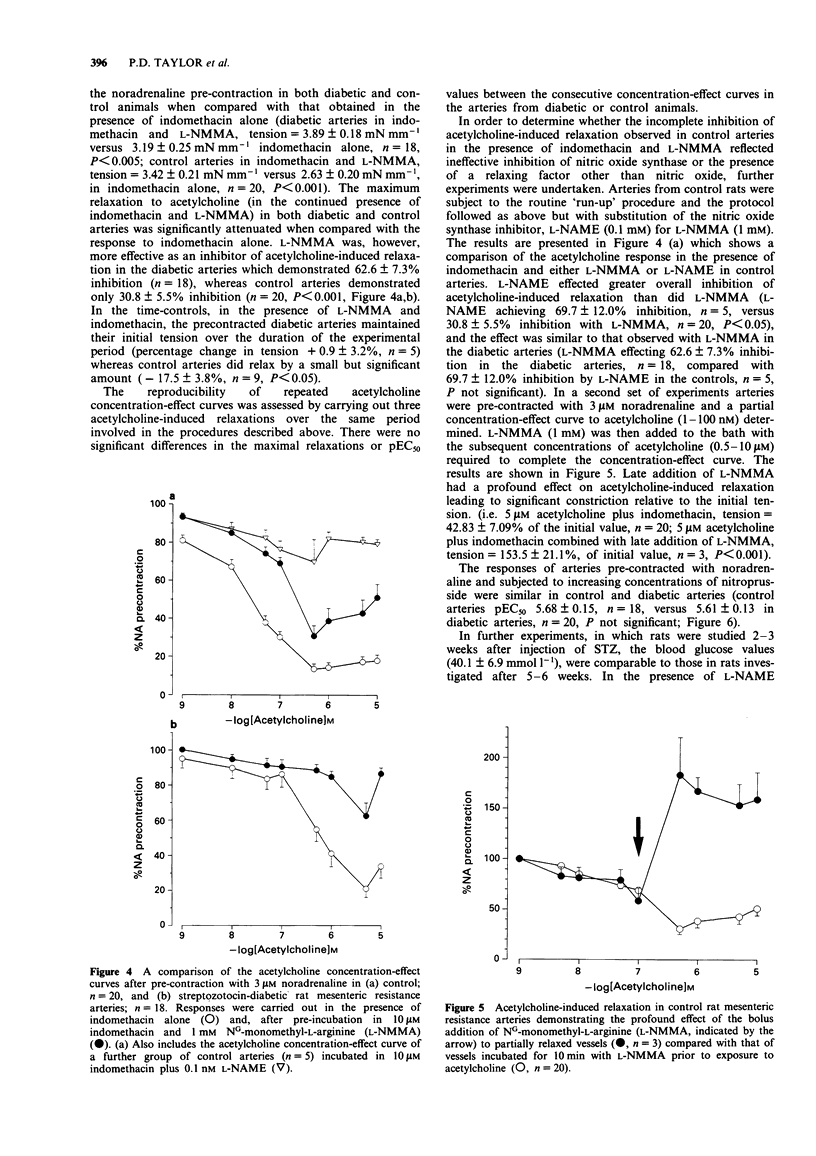


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abiru T., Kamata K., Miyata N., Kasuya Y. Differences in vascular responses to vasoactive agents of basilar artery and aorta from rabbits with alloxan-induced diabetes. Can J Physiol Pharmacol. 1990 Jul;68(7):882–888. doi: 10.1139/y90-134. [DOI] [PubMed] [Google Scholar]
- Arbogast B. W., Berry D. L., Newell C. L. Injury of arterial endothelial cells in diabetic, sucrose-fed and aged rats. Atherosclerosis. 1984 Apr;51(1):31–45. doi: 10.1016/0021-9150(84)90142-4. [DOI] [PubMed] [Google Scholar]
- Christlieb A. R., Janka H. U., Kraus B., Gleason R. E., Icasas-Cabral E. A., Aiello L. M., Cabral B. V., Solano A. Vascular reactivity to angiotensin II and to norepinephrine in diabetic subjects. Diabetes. 1976 Apr;25(4):268–274. doi: 10.2337/diab.25.4.268. [DOI] [PubMed] [Google Scholar]
- Cohen R. A., Tesfamariam B., Weisbrod R. M., Zitnay K. M. Adrenergic denervation in rabbits with diabetes mellitus. Am J Physiol. 1990 Jul;259(1 Pt 2):H55–H61. doi: 10.1152/ajpheart.1990.259.1.H55. [DOI] [PubMed] [Google Scholar]
- Dohi Y., Thiel M. A., Bühler F. R., Lüscher T. F. Activation of endothelial L-arginine pathway in resistance arteries. Effect of age and hypertension. Hypertension. 1990 Aug;16(2):170–179. doi: 10.1161/01.hyp.16.2.170. [DOI] [PubMed] [Google Scholar]
- Durante W., Sen A. K., Sunahara F. A. Impairment of endothelium-dependent relaxation in aortae from spontaneously diabetic rats. Br J Pharmacol. 1988 Jun;94(2):463–468. doi: 10.1111/j.1476-5381.1988.tb11548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D., Hadházy P., Koltai M. Z., Pogátsa G. Contractile and relaxant responses of diabetic dog femoral arteries. Acta Physiol Hung. 1988;71(2):213–217. [PubMed] [Google Scholar]
- Gebremedhin D., Koltai M. Z., Pogátsa G., Magyar K., Hadházy P. Altered responsiveness of diabetic dog renal arteries to acetylcholine and phenylephrine: role of endothelium. Pharmacology. 1989;38(3):177–184. doi: 10.1159/000138535. [DOI] [PubMed] [Google Scholar]
- Harris K. H., MacLeod K. M. Influence of the endothelium on contractile responses of arteries from diabetic rats. Eur J Pharmacol. 1988 Aug 9;153(1):55–64. doi: 10.1016/0014-2999(88)90587-0. [DOI] [PubMed] [Google Scholar]
- Head R. J., Longhurst P. A., Panek R. L., Stitzel R. E. A contrasting effect of the diabetic state upon the contractile responses of aortic preparations from the rat and rabbit. Br J Pharmacol. 1987 Jun;91(2):275–286. doi: 10.1111/j.1476-5381.1987.tb10282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M., Mitchell J. A., Harris H. J., Katsura M., Thiemermann C., Vane J. R. Endothelial cells metabolize NG-monomethyl-L-arginine to L-citrulline and subsequently to L-arginine. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1037–1043. doi: 10.1016/0006-291x(90)90627-y. [DOI] [PubMed] [Google Scholar]
- Kamata K., Miyata N., Kasuya Y. Impairment of endothelium-dependent relaxation and changes in levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br J Pharmacol. 1989 Jun;97(2):614–618. doi: 10.1111/j.1476-5381.1989.tb11993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappagoda T., Jayakody L., Rajotte R., Thomson A. B., Senaratne M. P. Endothelium-dependent relaxation to acetylcholine in the aorta of streptozotocin induced diabetic-rat and the BB-diabetic rat. Clin Invest Med. 1989 Jun;12(3):187–193. [PubMed] [Google Scholar]
- Kiff R. J., Gardiner S. M., Compton A. M., Bennett T. Selective impairment of hindquarters vasodilator responses to bradykinin in conscious Wistar rats with streptozotocin-induced diabetes mellitus. Br J Pharmacol. 1991 Jun;103(2):1357–1362. doi: 10.1111/j.1476-5381.1991.tb09793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiff R. J., Gardiner S. M., Compton A. M., Bennett T. The effects of endothelin-1 and NG-nitro-L-arginine methyl ester on regional haemodynamics in conscious rats with streptozotocin-induced diabetes mellitus. Br J Pharmacol. 1991 Jun;103(2):1321–1326. doi: 10.1111/j.1476-5381.1991.tb09787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod K. M., McNeill J. H. The influence of chronic experimental diabetes on contractile responses of rat isolated blood vessels. Can J Physiol Pharmacol. 1985 Jan;63(1):52–57. doi: 10.1139/y85-009. [DOI] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):529–538. [PubMed] [Google Scholar]
- Mayhan W. G. Impairment of endothelium-dependent dilatation of cerebral arterioles during diabetes mellitus. Am J Physiol. 1989 Mar;256(3 Pt 2):H621–H625. doi: 10.1152/ajpheart.1989.256.3.H621. [DOI] [PubMed] [Google Scholar]
- Mayhan W. G., Simmons L. K., Sharpe G. M. Mechanism of impaired responses of cerebral arterioles during diabetes mellitus. Am J Physiol. 1991 Feb;260(2 Pt 2):H319–H326. doi: 10.1152/ajpheart.1991.260.2.H319. [DOI] [PubMed] [Google Scholar]
- Meraji S., Jayakody L., Senaratne M. P., Thomson A. B., Kappagoda T. Endothelium-dependent relaxation in aorta of BB rat. Diabetes. 1987 Aug;36(8):978–981. doi: 10.2337/diab.36.8.978. [DOI] [PubMed] [Google Scholar]
- Mulhern M., Docherty J. R. Effects of experimental diabetes on the responsiveness of rat aorta. Br J Pharmacol. 1989 Aug;97(4):1007–1012. doi: 10.1111/j.1476-5381.1989.tb12555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Oyama Y., Kawasaki H., Hattori Y., Kanno M. Attenuation of endothelium-dependent relaxation in aorta from diabetic rats. Eur J Pharmacol. 1986 Dec 2;132(1):75–78. doi: 10.1016/0014-2999(86)90013-0. [DOI] [PubMed] [Google Scholar]
- Pieper G. M., Gross G. J. Oxygen free radicals abolish endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol. 1988 Oct;255(4 Pt 2):H825–H833. doi: 10.1152/ajpheart.1988.255.4.H825. [DOI] [PubMed] [Google Scholar]
- Porta M., La Selva M., Molinatti P., Molinatti G. M. Endothelial cell function in diabetic microangiopathy. Diabetologia. 1987 Aug;30(8):601–609. doi: 10.1007/BF00277315. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz de Tejada I., Goldstein I., Azadzoi K., Krane R. J., Cohen R. A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989 Apr 20;320(16):1025–1030. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- Tanz R. D., Chang K. S., Weller T. S. Histamine relaxation of aortic rings from diabetic rats. Agents Actions. 1989 Aug;28(1-2):1–8. doi: 10.1007/BF02022973. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B., Brown M. L., Cohen R. A. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Invest. 1991 May;87(5):1643–1648. doi: 10.1172/JCI115179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B., Brown M. L., Deykin D., Cohen R. A. Elevated glucose promotes generation of endothelium-derived vasoconstrictor prostanoids in rabbit aorta. J Clin Invest. 1990 Mar;85(3):929–932. doi: 10.1172/JCI114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B., Jakubowski J. A., Cohen R. A. Contraction of diabetic rabbit aorta caused by endothelium-derived PGH2-TxA2. Am J Physiol. 1989 Nov;257(5 Pt 2):H1327–H1333. doi: 10.1152/ajpheart.1989.257.5.H1327. [DOI] [PubMed] [Google Scholar]
- Wakabayashi I., Hatake K., Kimura N., Kakishita E., Nagai K. Modulation of vascular tonus by the endothelium in experimental diabetes. Life Sci. 1987 Feb 16;40(7):643–648. doi: 10.1016/0024-3205(87)90265-7. [DOI] [PubMed] [Google Scholar]
- Weidmann P., Beretta-Piccoli C., Keusch G., Glück Z., Mujagic M., Grimm M., Meier A., Ziegler W. H. Sodium-volume factor, cardiovascular reactivity and hypotensive mechanism of diuretic therapy in mild hypertension associated with diabetes mellitus. Am J Med. 1979 Nov;67(5):779–784. doi: 10.1016/0002-9343(79)90734-4. [DOI] [PubMed] [Google Scholar]
- White R. E., Carrier G. O. Enhanced vascular alpha-adrenergic neuroeffector system in diabetes: importance of calcium. Am J Physiol. 1988 Nov;255(5 Pt 2):H1036–H1042. doi: 10.1152/ajpheart.1988.255.5.H1036. [DOI] [PubMed] [Google Scholar]
- White R. E., Carrier G. O. Supersensitivity and endothelium dependency of histamine-induced relaxation in mesenteric arteries isolated from diabetic rats. Pharmacology. 1986;33(1):34–38. doi: 10.1159/000138197. [DOI] [PubMed] [Google Scholar]
- Woolfson R. G., Poston L. Effect of NG-monomethyl-L-arginine on endothelium-dependent relaxation of human subcutaneous resistance arteries. Clin Sci (Lond) 1990 Sep;79(3):273–278. doi: 10.1042/cs0790273. [DOI] [PubMed] [Google Scholar]


