Abstract
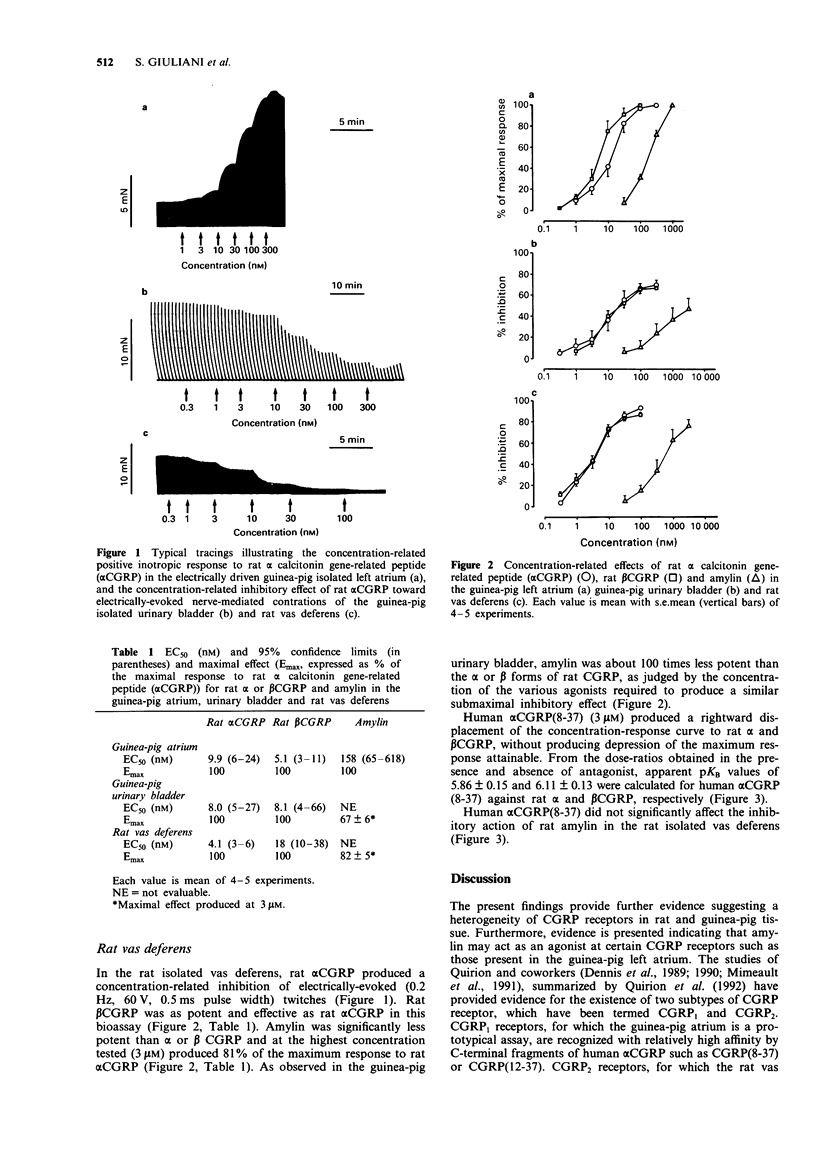
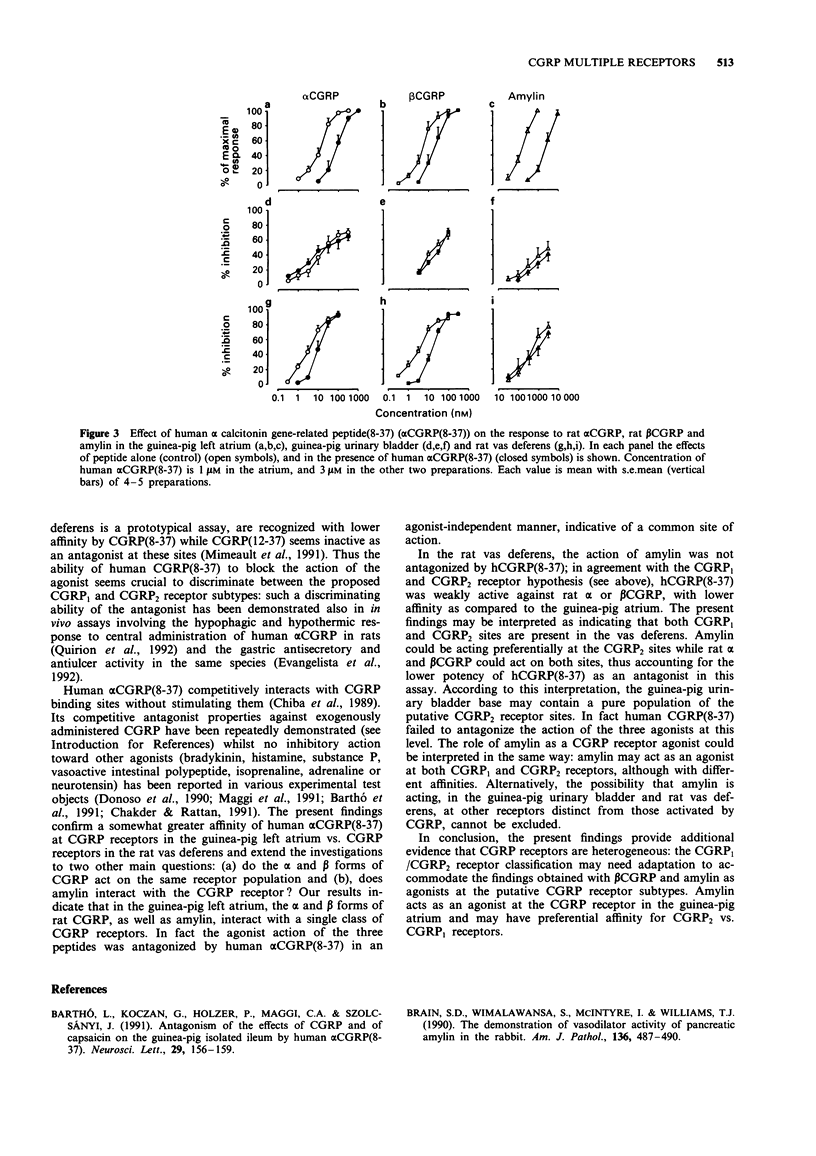
1. The activity of rat alpha and beta calcitonin gene-related peptide (CGRP) as compared to the structurally related peptide, rat amylin, has been investigated in the guinea-pig isolated left atrium (electrically driven), in mucosa-free strips from the base of the guinea-pig urinary bladder and in the rat isolated vas deferens (pars prostatica). The antagonist activity of the C-terminal fragment of human alpha CGRP, alpha CGRP(8-37), was also investigated. 2. In the guinea-pig isolated left atrium the three peptides produced a concentration-related positive inotropic effect, amylin being about 16 and 31 times less potent than alpha or beta CGRP, respectively. Human alpha CGRP(8-37) produced a rightward displacement of the log concentration-response curve to the three agonists tested, without depression of maximal response attainable. Apparent pKB values calculated on the basis of the displacement produced by 1 microM human alpha CGRP(8-37) indicated an agonist-independent affinity of the antagonist (6.66 +/- 0.11 for alpha CGRP, 6.42 +/- 0.17 for beta CGRP and 6.95 +/- 0.11 for amylin). 3. In the guinea-pig isolated urinary bladder, alpha or beta CGRP or amylin produce a concentration-related inhibition of twitch contractions evoked by train electrical field stimulation (10 Hz frequency, 0.25 ms duration at 100 V for 0.5 s every 60 s). Amylin was about 100 times less potent than alpha or beta CGRP. Human alpha CGRP(8-37) (3 microM) did not significantly affect the inhibitory action of the three agonists tested.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brain S. D., Wimalawansa S., MacIntyre I., Williams T. J. The demonstration of vasodilator activity of pancreatic amylin amide in the rabbit. Am J Pathol. 1990 Mar;136(3):487–490. [PMC free article] [PubMed] [Google Scholar]
- Breimer L. H., MacIntyre I., Zaidi M. Peptides from the calcitonin genes: molecular genetics, structure and function. Biochem J. 1988 Oct 15;255(2):377–390. doi: 10.1042/bj2550377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakder S., Rattan S. Antagonism of calcitonin gene-related peptide (CGRP) by human CGRP-(8-37): role of CGRP in internal anal sphincter relaxation. J Pharmacol Exp Ther. 1991 Mar;256(3):1019–1024. [PubMed] [Google Scholar]
- Chantry A., Leighton B., Day A. J. Cross-reactivity of amylin with calcitonin-gene-related peptide binding sites in rat liver and skeletal muscle membranes. Biochem J. 1991 Jul 1;277(Pt 1):139–143. doi: 10.1042/bj2770139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T., Yamaguchi A., Yamatani T., Nakamura A., Morishita T., Inui T., Fukase M., Noda T., Fujita T. Calcitonin gene-related peptide receptor antagonist human CGRP-(8-37). Am J Physiol. 1989 Feb;256(2 Pt 1):E331–E335. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Day A. J., Willis A. C., Roberts A. N., Reid K. B., Leighton B. Amylin and the amylin gene: structure, function and relationship to islet amyloid and to diabetes mellitus. Biochim Biophys Acta. 1989 Dec 14;1014(3):247–258. doi: 10.1016/0167-4889(89)90220-6. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Willis A. C., Clark A., Turner R. C., Sim R. B., Reid K. B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta H. K., Zaidi M., Wimalawansa S. J., Ghatei M. A., Beacham J. L., Bloom S. R., MacIntyre I. In vivo and in vitro effects of amylin and amylin-amide on calcium metabolism in the rat and rabbit. Biochem Biophys Res Commun. 1989 Jul 31;162(2):876–881. doi: 10.1016/0006-291x(89)92391-7. [DOI] [PubMed] [Google Scholar]
- Dennis T., Fournier A., Cadieux A., Pomerleau F., Jolicoeur F. B., St Pierre S., Quirion R. hCGRP8-37, a calcitonin gene-related peptide antagonist revealing calcitonin gene-related peptide receptor heterogeneity in brain and periphery. J Pharmacol Exp Ther. 1990 Jul;254(1):123–128. [PubMed] [Google Scholar]
- Dennis T., Fournier A., St Pierre S., Quirion R. Structure-activity profile of calcitonin gene-related peptide in peripheral and brain tissues. Evidence for receptor multiplicity. J Pharmacol Exp Ther. 1989 Nov;251(2):718–725. [PubMed] [Google Scholar]
- Donoso M. V., Fournier A., St-Pierre S., Huidobro-Toro J. P. Pharmacological characterization of CGRP1 receptor subtype in the vascular system of the rat: studies with hCGRP fragments and analogs. Peptides. 1990 Sep-Oct;11(5):885–889. doi: 10.1016/0196-9781(90)90003-n. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T., Bose C., Foulkes R., Hughes B. Antagonistic effect of human alpha-calcitonin gene-related peptide (8-37) on regional hemodynamic actions of rat islet amyloid polypeptide in conscious Long-Evans rats. Diabetes. 1991 Aug;40(8):948–951. doi: 10.2337/diab.40.8.948. [DOI] [PubMed] [Google Scholar]
- Giuliani S., Maggi C. A., Meli A. Prejunctional modulatory action of neuropeptide Y on peripheral terminals of capsaicin-sensitive sensory nerves. Br J Pharmacol. 1989 Oct;98(2):407–412. doi: 10.1111/j.1476-5381.1989.tb12611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani S., Santicioli P., Tramontana M., Geppetti P., Maggi C. A. Peptide N-formyl-methionyl-leucyl-phenylalanine (FMLP) activates capsaicin-sensitive primary afferent nerves in guinea-pig atria and urinary bladder. Br J Pharmacol. 1991 Mar;102(3):730–734. doi: 10.1111/j.1476-5381.1991.tb12241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida-Yamamoto A., Tohyama M. Calcitonin gene-related peptide in the nervous tissue. Prog Neurobiol. 1989;33(5-6):335–386. doi: 10.1016/0301-0082(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Le Grevès P., Nyberg F., Hökfelt T., Terenius L. Calcitonin gene-related peptide is metabolized by an endopeptidase hydrolyzing substance P. Regul Pept. 1989 Jun-Jul;25(3):277–286. doi: 10.1016/0167-0115(89)90176-6. [DOI] [PubMed] [Google Scholar]
- Leighton B., Cooper G. J. Pancreatic amylin and calcitonin gene-related peptide cause resistance to insulin in skeletal muscle in vitro. Nature. 1988 Oct 13;335(6191):632–635. doi: 10.1038/335632a0. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Chiba T., Giuliani S. Human alpha-calcitonin gene-related peptide-(8-37) as an antagonist of exogenous and endogenous calcitonin gene-related peptide. Eur J Pharmacol. 1991 Jan 3;192(1):85–88. doi: 10.1016/0014-2999(91)90072-x. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Giuliani S. The neurotransmitter role of calcitonin gene-related peptide in the rat and guinea-pig ureter: effect of a calcitonin gene-related peptide antagonist and species-related differences in the action of omega conotoxin on calcitonin gene-related peptide release from primary afferents. Neuroscience. 1991;43(1):261–268. doi: 10.1016/0306-4522(91)90433-o. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Santicioli P., Patacchini R., Geppetti P., Giuliani S., Astolfi G. M., Baldi E., Parlani M., Theodorsson E., Fusco B. Regional differences in the motor response to capsaicin in the guinea-pig urinary bladder: relative role of pre- and postjunctional factors related to neuropeptide-containing sensory nerves. Neuroscience. 1988 Nov;27(2):675–688. doi: 10.1016/0306-4522(88)90297-7. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Theodorsson E., Santicioli P., Giuliani S. Tachykinins and calcitonin gene-related peptide as co-transmitters in local motor responses produced by sensory nerve activation in the guinea-pig isolated renal pelvis. Neuroscience. 1992;46(3):549–559. doi: 10.1016/0306-4522(92)90143-p. [DOI] [PubMed] [Google Scholar]
- Mimeault M., Fournier A., Dumont Y., St-Pierre S., Quirion R. Comparative affinities and antagonistic potencies of various human calcitonin gene-related peptide fragments on calcitonin gene-related peptide receptors in brain and periphery. J Pharmacol Exp Ther. 1991 Sep;258(3):1084–1090. [PubMed] [Google Scholar]
- Poyner D. R., Andrew D. P., Brown D., Bose C., Hanley M. R. Pharmacological characterization of a receptor for calcitonin gene-related peptide on rat, L6 myocytes. Br J Pharmacol. 1992 Feb;105(2):441–447. doi: 10.1111/j.1476-5381.1992.tb14272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Hayden D. W., O'Brien T. D., Johnson K. H. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]


