Abstract
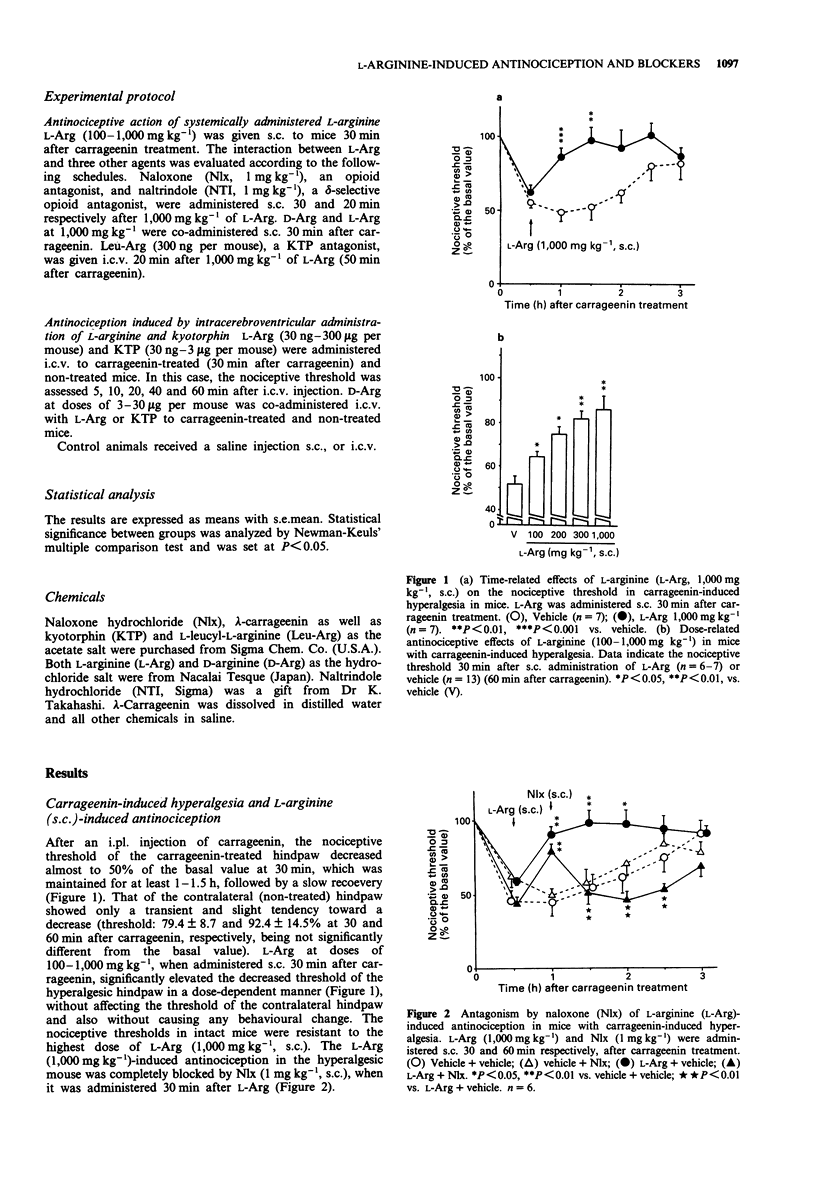
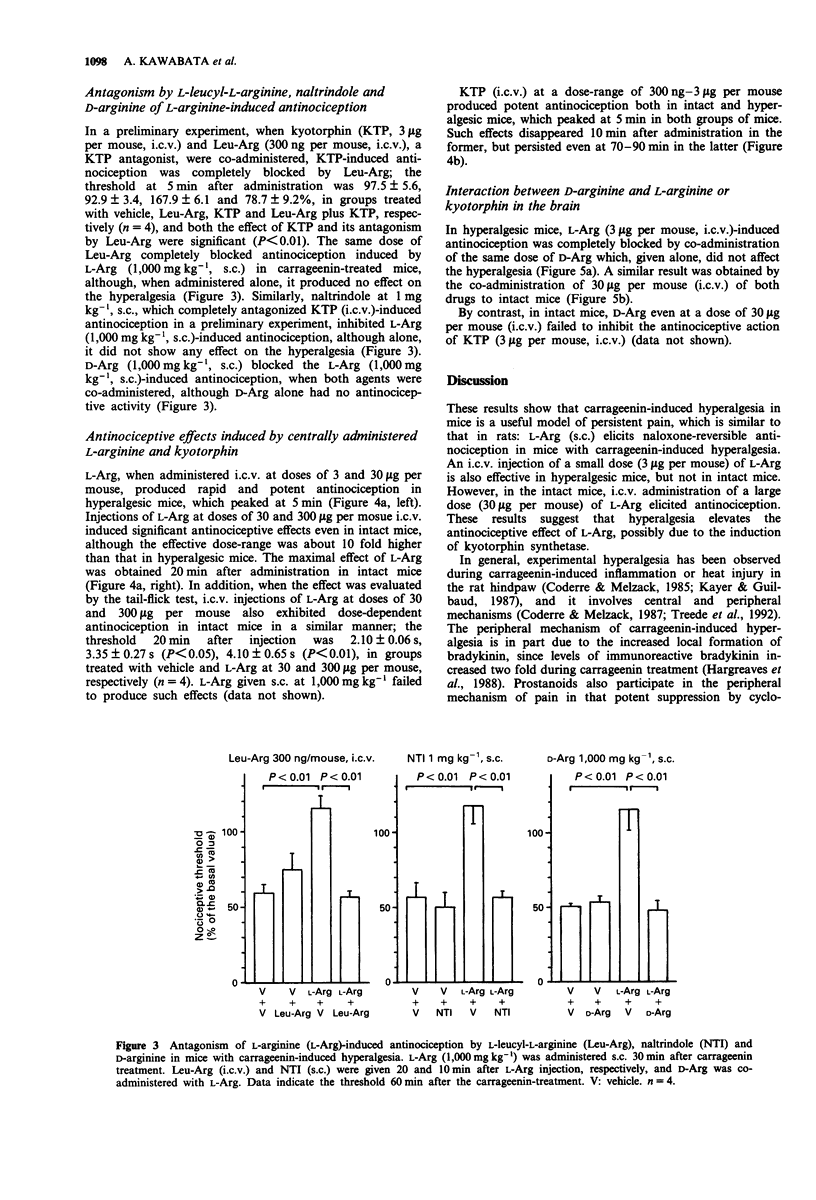
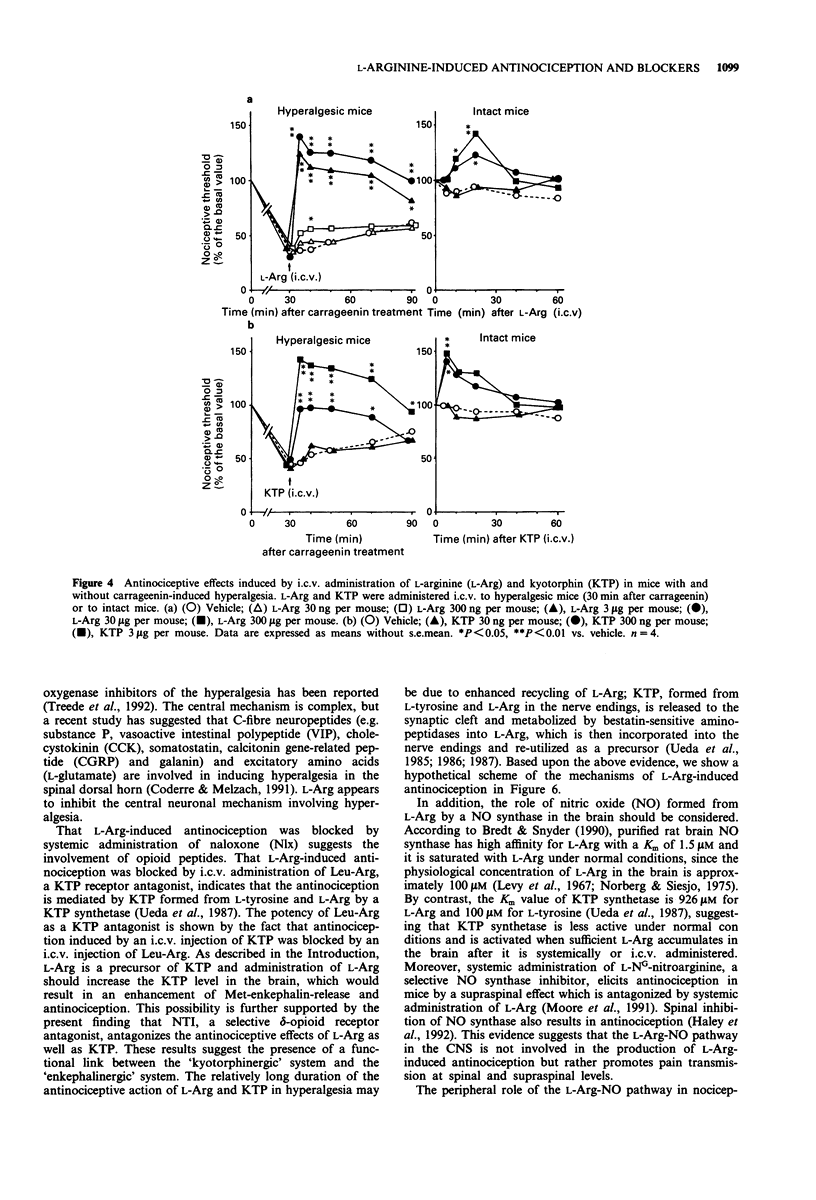
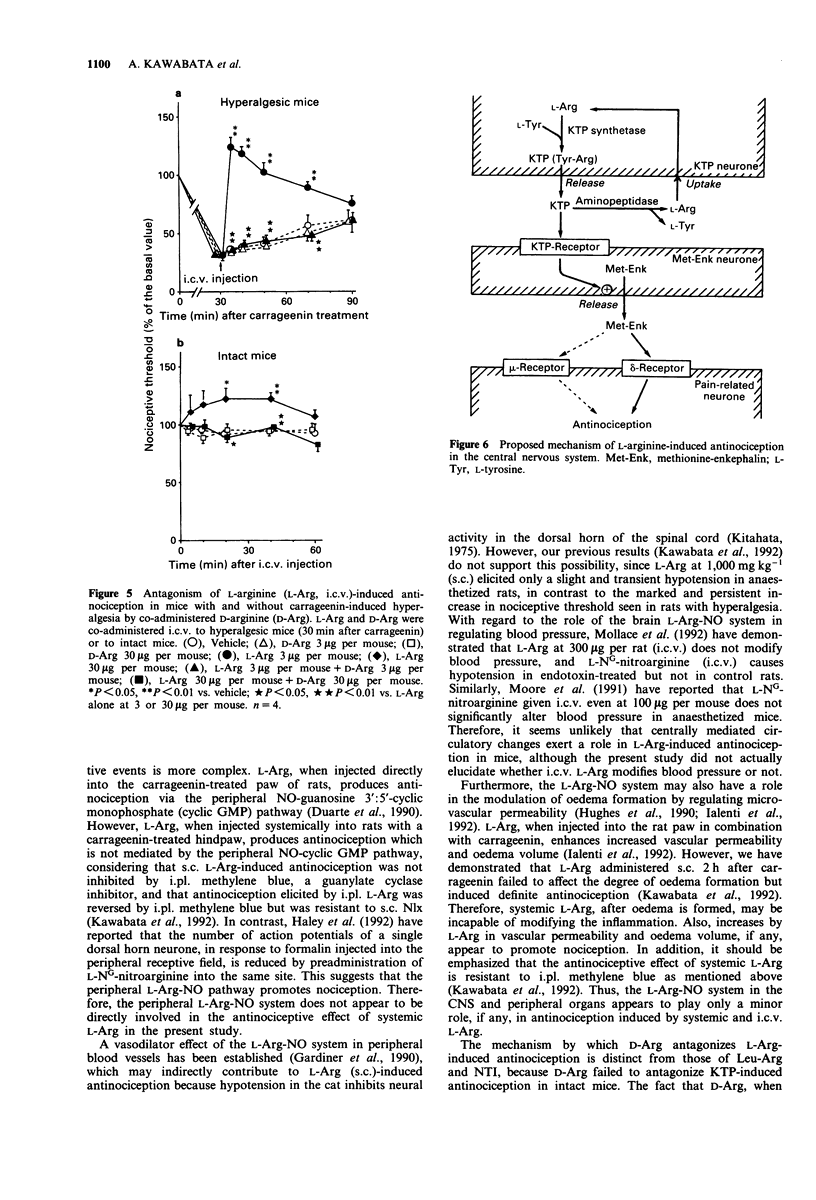
1. Intraplantar injection of carrageenin into the mouse hind paw produced hyperalgesia when measured by the paw pressure test (Randall & Selitto method). 2. Subcutaneous administration of L-arginine (100-1,000 mg kg-1), a possible precursor of kyotorphin which is an endogenous analgesic neuropeptide, inhibited carrageenin-induced hyperalgesia in a dose-dependent manner. This effect was blocked by subcutaneous administration of naloxone, naltrindole, a selective delta-opioid receptor antagonist (enkephalin antagonist), and D-arginine. 3. Intracerebroventricular administration of L-leucyl-L-arginine inhibited the antinociceptive effect of systemically administered L-arginine in hyperalgesic mice. 4. Intracerebroventricular administration of L-arginine (3 and 30 micrograms per mouse) and kyotorphin (300 ng-3 micrograms per mouse) produced antinociception in hyperalgesic mice. The antinociceptive effects of L-arginine but not kyotorphin were blocked by intracerebroventricular administration of D-arginine. 5. These results suggest that L-arginine-induced antinociception is mediated by activation of 'kyotorphinergic' nerves followed by activation of the 'opioidergic' (possible 'enkephalinergic') nerves in the central nervous system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre T. J., Melzack R. Central neural mediators of secondary hyperalgesia following heat injury in rats: neuropeptides and excitatory amino acids. Neurosci Lett. 1991 Sep 30;131(1):71–74. doi: 10.1016/0304-3940(91)90339-u. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Melzack R. Cutaneous hyperalgesia: contributions of the peripheral and central nervous systems to the increase in pain sensitivity after injury. Brain Res. 1987 Feb 24;404(1-2):95–106. doi: 10.1016/0006-8993(87)91359-x. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Melzack R. Increased pain sensitivity following heat injury involves a central mechanism. Behav Brain Res. 1985 May;15(3):259–262. doi: 10.1016/0166-4328(85)90181-0. [DOI] [PubMed] [Google Scholar]
- Durate I. D., Lorenzetti B. B., Ferreira S. H. Peripheral analgesia and activation of the nitric oxide-cyclic GMP pathway. Eur J Pharmacol. 1990 Sep 21;186(2-3):289–293. doi: 10.1016/0014-2999(90)90446-d. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. Regional haemodynamic changes during oral ingestion of NG-monomethyl-L-arginine or NG-nitro-L-arginine methyl ester in conscious Brattleboro rats. Br J Pharmacol. 1990 Sep;101(1):10–12. doi: 10.1111/j.1476-5381.1990.tb12079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley J. E., Dickenson A. H., Schachter M. Electrophysiological evidence for a role of nitric oxide in prolonged chemical nociception in the rat. Neuropharmacology. 1992 Mar;31(3):251–258. doi: 10.1016/0028-3908(92)90175-o. [DOI] [PubMed] [Google Scholar]
- Hargreaves K. M., Troullos E. S., Dionne R. A., Schmidt E. A., Schafer S. C., Joris J. L. Bradykinin is increased during acute and chronic inflammation: therapeutic implications. Clin Pharmacol Ther. 1988 Dec;44(6):613–621. doi: 10.1038/clpt.1988.202. [DOI] [PubMed] [Google Scholar]
- Harima A., Shimizu H., Takagi H. Analgesic effect of L-arginine in patients with persistent pain. Eur Neuropsychopharmacol. 1991 Dec;1(4):529–533. doi: 10.1016/0924-977x(91)90006-g. [DOI] [PubMed] [Google Scholar]
- Hughes S. R., Williams T. J., Brain S. D. Evidence that endogenous nitric oxide modulates oedema formation induced by substance P. Eur J Pharmacol. 1990 Dec 4;191(3):481–484. doi: 10.1016/0014-2999(90)94184-y. [DOI] [PubMed] [Google Scholar]
- Ialenti A., Ianaro A., Moncada S., Di Rosa M. Modulation of acute inflammation by endogenous nitric oxide. Eur J Pharmacol. 1992 Feb 11;211(2):177–182. doi: 10.1016/0014-2999(92)90526-a. [DOI] [PubMed] [Google Scholar]
- Kawabata A., Fukuzumi Y., Fukushima Y., Takagi H. Antinociceptive effect of L-arginine on the carrageenin-induced hyperalgesia of the rat: possible involvement of central opioidergic systems. Eur J Pharmacol. 1992 Jul 21;218(1):153–158. doi: 10.1016/0014-2999(92)90159-2. [DOI] [PubMed] [Google Scholar]
- Kayser V., Guilbaud G. Local and remote modifications of nociceptive sensitivity during carrageenin-induced inflammation in the rat. Pain. 1987 Jan;28(1):99–107. doi: 10.1016/0304-3959(87)91064-5. [DOI] [PubMed] [Google Scholar]
- Kitahata L. M. Modes and sites of "analgesic" action of anesthetics on the spinal cord. Int Anesthesiol Clin. 1975 Spring;13(1):149–170. doi: 10.1097/00004311-197513010-00007. [DOI] [PubMed] [Google Scholar]
- Levi G., Kandera J., Lajtha A. Control of cerebral metabolite levels. I. Amino acid uptake and levels in various species. Arch Biochem Biophys. 1967 Mar;119(1):303–311. doi: 10.1016/0003-9861(67)90460-2. [DOI] [PubMed] [Google Scholar]
- Levy L. Carrageenan paw edema in the mouse. Life Sci. 1969 Jun 1;8(11):601–606. doi: 10.1016/0024-3205(69)90021-6. [DOI] [PubMed] [Google Scholar]
- Mollace V., De Francesco E. A., Nisticó G. Evidence that pharmacological manipulations of central L-arginine-NO pathway influence blood pressure and heart rate in rats. Neurosci Lett. 1992 Mar 16;137(1):87–90. doi: 10.1016/0304-3940(92)90305-q. [DOI] [PubMed] [Google Scholar]
- Moore P. K., Oluyomi A. O., Babbedge R. C., Wallace P., Hart S. L. L-NG-nitro arginine methyl ester exhibits antinociceptive activity in the mouse. Br J Pharmacol. 1991 Jan;102(1):198–202. doi: 10.1111/j.1476-5381.1991.tb12153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoghese P. S., Sultana M., Takemori A. E. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol. 1988 Jan 27;146(1):185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- RANDALL L. O., SELITTO J. J. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957 Sep 1;111(4):409–419. [PubMed] [Google Scholar]
- Takagi H., Shiomi H., Ueda H., Amano H. A novel analgesic dipeptide from bovine brain is a possible Met-enkephalin releaser. Nature. 1979 Nov 22;282(5737):410–412. doi: 10.1038/282410a0. [DOI] [PubMed] [Google Scholar]
- Takagi H., Shiomi H., Ueda H., Amano H. Morphine-like analgesia by a new dipeptide, L-tyrosyl-L-arginine (Kyotorphin) and its analogue. Eur J Pharmacol. 1979 Apr 1;55(1):109–111. doi: 10.1016/0014-2999(79)90154-7. [DOI] [PubMed] [Google Scholar]
- Treede R. D., Meyer R. A., Raja S. N., Campbell J. N. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38(4):397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Ueda H., Matsumoto S., Yoshihara Y., Fukushima N., Takagi H. Uptake and release of kyotorphin in rat brain synaptosomes. Life Sci. 1986 Jun 30;38(26):2405–2411. doi: 10.1016/0024-3205(86)90609-0. [DOI] [PubMed] [Google Scholar]
- Ueda H., Ming G., Hazato T., Katayama T., Takagi H. Degradation of kyotorphin by a purified membrane-bound-aminopeptidase from monkey brain: potentiation of kyotorphin-induced analgesia by a highly effective inhibitor, bestatin. Life Sci. 1985 May 13;36(19):1865–1871. doi: 10.1016/0024-3205(85)90160-2. [DOI] [PubMed] [Google Scholar]
- Ueda H., Tatsumi K., Shiomi H., Takagi H. Analgesic dipeptide, kyotorphin (Tyr-Arg), is highly concentrated in the synaptosomal fraction of the rat brain. Brain Res. 1982 Jan 7;231(1):222–224. doi: 10.1016/0006-8993(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Ueda H., Yoshihara Y., Fukushima N., Shiomi H., Nakamura A., Takagi H. Kyotorphin (tyrosine-arginine) synthetase in rat brain synaptosomes. J Biol Chem. 1987 Jun 15;262(17):8165–8173. [PubMed] [Google Scholar]
- Ueda H., Yoshihara Y., Misawa H., Fukushima N., Katada T., Ui M., Takagi H., Satoh M. The kyotorphin (tyrosine-arginine) receptor and a selective reconstitution with purified Gi, measured with GTPase and phospholipase C assays. J Biol Chem. 1989 Mar 5;264(7):3732–3741. [PubMed] [Google Scholar]
- Yoshihara Y., Ueda H., Imajoh S., Takagi H., Satoh M. Calcium-activated neutral protease (CANP), a putative processing enzyme of the neuropeptide, kyotorphin, in the brain. Biochem Biophys Res Commun. 1988 Sep 15;155(2):546–553. doi: 10.1016/s0006-291x(88)80529-1. [DOI] [PubMed] [Google Scholar]


